Archives of Hepatitis Research
Liver Specific Serum Micro RNA122 as a Prognostic Marker in Egyptian Patients with Liver Cirrhosis
Mona A Amin1*, Mai Fawzi1, Dina Sabri2, Heba Sedrak1, Shrouk Mausa1 and Abdelaziz El-Saadi1
2Department of Medical biochemistry, Kasr Al-Aini, Cairo University, Egypt
Cite this as
Amin MA, Fawzi M, Sabri D, Sedrak H, Mausa S, et al. (2017) Liver Specific Serum Micro RNA122 as a Prognostic Marker in Egyptian Patients with Liver Cirrhosis. Archives of Hepatitis Research 3(1): 004-009. DOI: 10.17352/ahr.000008Introduction: Recent research has shown that microRNAs (miRNA) are emerging as important regulators of cellular differentiation. The miR-122 accounts for approximately 70% of all miRNAs in the liver so its presence in the serum is highly indicative of liver processes.
Aim of the work and methods: was to study the role of miR-122 as a prognostic new marker in patients with liver cirrhosis. MiR-122 was detected by quantitative real-time reverse transcription (RT)-PCR technique. Eighty patients with liver cirrhosis were included in our study, we divided them into 4 equal groups according to the complications of liver cirrhosis (1-compensated cirrhotics, 2- cirrhotics with ascites, 3-spontaneous bacterial peritonitis (SBP), and 4- hepatorenal syndrome (HRS) group) to evaluate if the serum level of miR-122 might be a suitable parameter for assessment of disease severity and prognosis in such patients.
Results: miR-122 was statistically significantly higher in group 1 “compensated” when compared to both groups 2 “ascites” and 3 “SBP” (P=0.001), while the difference was highly significant when compared to its level in group 4 ‘’HRS’’ (P<0.001). Serum miR-122 levels were positively correlated with serum albumin, PC, and serum Na levels while it was negatively correlated with creatinine, urea, and INR. Also there was strong negative correlation between serum miR-122 level and both MELD and Child score.
Conclusion: Lower serum miR-122 levels are associated with ascites, spontaneous bacterial peritonitis and hepatorenal syndrome. Therefore, serum miR-122 could be a new potential parameter and a prognostic marker in patients with liver cirrhosis.
Introduction
Liver cirrhosis is a major health problem worldwide and is associated with significant morbidity and mortality. According to the WHO about 800,000 people die of cirrhosis annually. It has a significant impact on the economy as a result of premature death, illness, and disability [1]. The patient’s prognosis worsens with the occurrence of liver cirrhosis complications such as ascites, SBP or HRS [2]. Ascites is a major complication of cirrhosis and is associated with 50% mortality over two years [3]. Hepatorenal syndrome (HRS) a serious complication occurring mainly in patients with advanced cirrhosis and ascites, carries a grave prognosis [4]. Spontaneous bacterial peritonitis (SBP) is a complication of ascites in decompensated liver cirrhosis and is associated with a mortality rate exceeding 90% if untreated, while with early diagnosis and prompt treatment the mortality rate dropped to 20% [5].
Poor survival of patients with decompensated cirrhosis has been a driving force for physicians to search for markers that provide clues to the presence of decompensation and the prognosis of these patients. The Child-Pugh (CTP) score had been introduced to assess the prognosis of patients with cirrhosis, since it is related to the severity of the liver disease [6]. Unfortunately, the score was found to have a “ceiling effect”; it is able to differentiate disease severity only in markedly decompensated cirrhotic patients [7]. The Model of End Stage Liver Disease (MELD) score was originally developed to predict survival after Transjugular Intrahepatic Portosystemic shunt procedure and to prioritize patients waiting for liver transplantation [2]. It has been found to predict with accuracy short term mortality in various liver diseases [8]. However, the MELD score doesn’t account for the presence of some complications of liver cirrhosis as ascites and encephalopathy which can be viewed as a shortcoming and it is unable to give accurate predictions for long term survival [9]. Moreover, on comparing the two scores, results are sometimes conflicting. Therefore, the evaluation of new markers is an important task in patients with liver cirrhosis [10].
MicroRNAs (miRNAs, miRs) are small (18–25 ribonucleotides) non-coding RNAs which function in transcriptional and post-transcriptional regulation of gene expression [11]. Circulating miRs are,in large part, derived from cells with damaged plasma membrane [12] and circulate in the blood freely and in a relatively stable form [13,14]. Circulating miRs are found in lipid or lipoprotein complexes providing an association between plasma miR levels and specific organ dysfunction [15]. miRs modulate diverse cellular processes associated with liver injury as inflammation, apoptosis, and hepatocyte regeneration [16]. The miR-122 is liver specific and accounts for approximately 70% of all miRs in the liver [15]. Studies revealed a role for miRNA-122 in hepatic stellate cell expression and thus liver inflammation and fibrosis miR-122 level has an inverse effect on liver fibrosis by targeting the gene that encodes transmembrane propyl 4 hydroxylase which is involved in collagen maturation [17]. Elevated levels of the liver-specific miR-122 have been found in sera or plasma of patients with chronic hepatitis B infection [18,19]. As well as in sera or plasma from humans and rodents upon toxic liver injury [20-22]. Moreover, circulating miR-122 has been proposed as a marker of inflammation in patients with chronic hepatitis C viral infection [23].
Aim of the work
Was to study the role of miR-122 as a new prognostic marker in patients with compensated and decompensated liver cirrhosis with different complications of liver cirrhosis and relate that to other scoring systems.
Patients and Methods
Study population and selection of patients
After obtaining the approval of the Ethics committee for the Faculty of Medicine, Cairo University, 80 patients with liver cirrhosis due to hepatitis C virus infection above the age of 18 were enrolled in the study. They were referred to the inpatient ward and outpatient clinics of the department of Internal Medicine at Kasr Al-Aini hospital during the period between January and May 2014. All patients gave an informed, written consent. The diagnosis of liver cirrhosis was based on well-established clinical, laboratory and ultrasonographic features. The diagnosis of ascites was confirmed by ultrasonography. Paracentesis was performed if appropriate amounts of ascites were detectable. The diagnosis of spontaneous bacterial peritonitis was based on a neutrophil count >250 cell/ mm\ in an ascites fluid sample and/or a positive ascitic fluid culture. Hepatorenal syndrome was diagnosed according to the EASL clinical practice guidelines [22].
Exclusion criteria included patients with liver cirrhosis caused by hepatitis B virus or hepatitis B and C co-infection, patients with hepatocellular carcinoma, patients who underwent liver transplantation, patients diagnosed with Budd-Chiari syndrome and patients with portal vein thrombosis.
Our patients were divided into four equal groups
Group I were those with compensated liver cirrhosis, group II cirrhotic patients with ascites, group III cirrhotic patients with SBP and group IV cirrhotic patients with HRS.
Blood sampling
Peripheral blood was collected from each individual at the day of enrollment into the study. The serum tubes were centrifuged at 1500× g for 10 min at 4°C, followed by an additional centrifugation step at 2000× g 4°C to completely remove any remaining cells. The serum samples were aliquoted and stored at −80°C until further use.
Clinical chemistry
1- Standard parameters of liver and kidney function were measured.
2- Calculating of CTP [22] and MELD scores (MELD score was calculated according to the standard formula as follows: 11.2 × ln (INR) + 9.57 × ln (creatinine, in milligrams per deciliter) + 3.78 × ln (bilirubin, in milligrams per deciliter) + 6.43 [7].
3- Detection of miRs by quantitative real-time reverse-transcription (RT)-PCR:
The mirVana™ miR isolation kit was designed for purification of RNA suitable for studies of miR in natural populations. The kit employs an organic extraction followed by immobilization of RNA on glass-fiber filters to purify either total RNA or RNA enriched for small species from cells or tissue samples.
Procedure of the mirVana miRNA Isolation kit
By the Chemical extraction and immobilization method; highly concentrated chaotropic salts in conjunction with acidic phenol or phenol-chloroform solutions are used to inactivate RNases and purify RNA from other biomolecules. This method provided very pure preparations of RNA; however, the RNA must typically be desalted and concentrated with an alcohol precipitation step. Routine alcohol precipitation does not quantitatively recover small nucleic acid molecules, making it ill-suited for the preparation of very small RNAs enriched for small species from cells or tissue samples.
Statistical analysis
Descriptive statistical are described in term of mean, standard deviation, and percentage. For comparing categorical data Chi square test performed. The data analyzed by using SPSS (Statistical Package for the social science) version 20 for Microsoft Windows. Statistical differences among groups were tested by using independent one way ANOVA test (Analysis of Variance) for repeated measurements followed by post-hoc comparison with Tukey HSD test. Bonferroni correlation was used when multiple subgroup comparisons were performed. P value of < 0.05 was considered to be significant.
Results
Table 1 shows the patient characteristics of all studied groups. Among the eighty patients studied, 56 was males (70%) and 24 was females (30%), and the mean age was (55.2 ± 8.2).
There was highly statistically significant difference in serum albumin, PC, total bilirubin, serum creatinine, serum sodium and potassium levels between the “compensated” group and the other three groups.
There was a statistical difference between the compensated group and the decompensated groups with regard to the Child-Pugh score as well as the MELD score that was highly significant. (P<0.001).
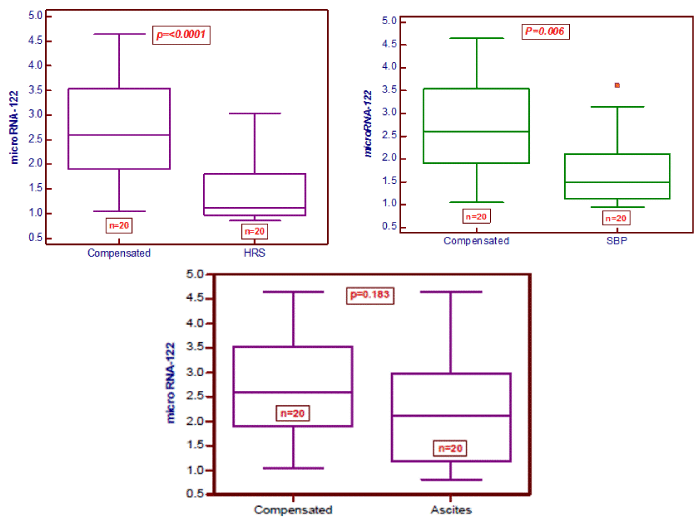
Serum miR-122 levels are associated with decompensation of liver cirrhosis
Serum levels of miR-122 in patients with hepatic decompensation were significantly lower when compared to patients with compensated liver disease (P<0.001). Patients with SBP (P=0.006) or HRS (P<0.0001) had significantly lower serum levels of miR-122 than patients without the respective complication. In contrast, no significant differences were observed between liver cirrhosis patients with and without ascites (P=0.183) Figure 1.
Serum miR-122 levels correlate with parameters of liver damage and hepatic functional capacity
Correlation studies of CTP score with other parameters revealed the strong positive correlation of CTP score with INR, serum Creatinine, urea, total bilirubin, and PT, while a strong negative correlation was found with serum albumin and sodium Table 2.
Correlation between the MELD score and other parameters revealed a strong positive correlation of MELD score with INR, serum creatinine, urea, total bilirubin and PT whereas it revealed a strong negative correlation with serum albumin and Na Table 3.
Correlation between serum miR-122 level and other parameters revealed a strong positive correlation of miR-122 with serum albumin, PC, and sodium while it revealed a strong negative correlation with serum creatinine, urea, INR and K Table 4.
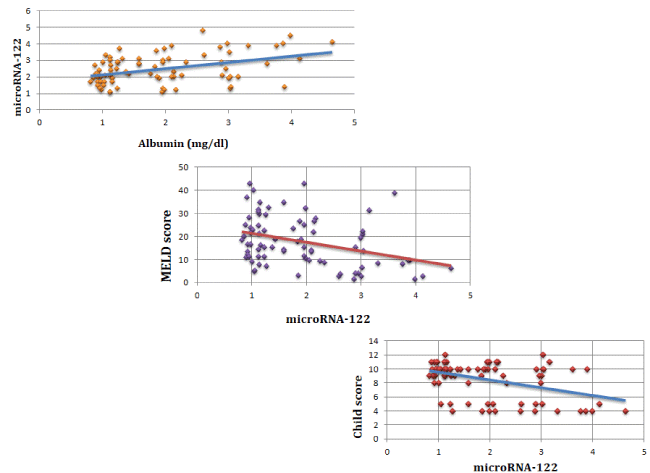
Serum miR-122 correlates with child and MELD scores
The correlation between serum miR-122 level and both MELD and Child scores is shown in Figure 2. There was a strong negative correlation between serum miR-122 level and MELD score (p=0.001), and very a strong negative correlation between serum miR-122 level and Child score (p <0.001).
There was a very strong positive correlation between serum miR-122 level and serum albumin level (P = 0.0002).
Backward stepwise multivariate linear regression was conducted to explore the predictors of Micro-RNA by using MELD Score, Child- Score and Albumin, it revealed that albumin was the only significant independent predictor Table 5.
Discussion
In the era of direct antiviral drugs, many patients with viral related liver cirrhosis are expected avoid the long term morbidity of previous decades. But in countries where the brunt of viral hepatitis affected a significant percentage of its population cirrhosis and its complications continue to be a health problem. A recent consensus has proposed that compensated and decompensated cirrhosis be considered separate disease entities [24]. The survival rate of patients with decompensated liver cirrhosis is less than two years which is much worse than patients with compensated liver disease whose median survival is more than 12 years [3,25]. The Child Pugh score and the MELD score were formulated to guide clinicians in their assessment of decompensation. However, their limitations have led to the incorporation of additional parameters to improve prognostic accuracy [26]. Furthermore the MELD score does not correlate well with the severity of hepatic encephalopathy or ascites. Patients with encephalopathy or abnormal EEG and suggestive neuropsychometric tests have a MELD score that is less than 25 in 90% of cases [27].
The aim of this work was to assess the usefulness of miRNA-122 as a prognostic marker of decompensation in liver cirrhosis. Our data showed that miR-122 serum levels were statistically significantly lower in patients with SBP and HRS than those with compensated cirrhosis or cirrhosis with ascites. Our study also found a strong negative correlation between miR-122 and both the MELD and Child Pugh scores.
The laboratory parameters of the patients with decompensated cirrhosis showed abnormal values particularly those related to the synthetic capacity of the liver. This was more pronounced in the groups with SBP and HRS. The Child Pugh and MELD scores are partially based on some of these parameters. Our data showed a significant difference between the four groups in the Child Pugh score. This means the Child Pugh score correlates well with decompensation and its complications. Our results confirm those of Khot et al., who demonstrated that the Child Pugh score was a significant predictor of mortality [28]. Takaya et al., also concluded that patients with a Child Pugh score in the C range have worse survival [29]. The MELD score was significantly different between the four groups of the study with higher values found in the group with SBP while the highest values were obtained from the group with HRS. Licata et al., also found that patients with HRS had high MELD scores [30]. There was a significant difference in the level of serum sodium between our groups which matches the results described by Zhang et al., who concluded that the serum sodium concentration decreases in conjunction with the level of severity in decompensated cirrhosis [31]. Significantly lower levels of serum sodium in patients with HRS has also been mentioned in other studies [30,32]. Our findings are in concordance with the study done by Shaikh et al., who demonstrated that hyponatremia has a negative influence on cirrhosis related complications [33].
The serum levels of miR-122 were significantly lower in the group with SBP and HRS patients than in those without complications. Waidmann et al., showed a similar decrement of miR-122 in SBP and HRS than compensated cirrhotic patients [34].
Both the Child Pugh score and the MELD score correlated positively with T. bilirubin, INR, creatinine and urea. Both scores also showed a strong negative correlation with albumin and sodium. The MELD score was strongly positively correlated with creatinine and urea, and these results are confirmed by the work done by Sumskiene et al., who found statistically significant low survival in patients with high serum bilirubin, high serum creatinine and high blood urea [35]. These correlations assert the role of these scores as significant and reliable prognostic predictors of morbidity in decompensated cirrhosis, especially in patients with SBP and HRS. Early prognostic markers of severity might improve the outcome and reduce mortality related to complications of decompensated cirrhosis. Circulating miR-122 exhibited a strong negative correlation with T. bilirubin, creatinine, urea and a strong positive correlation with PC, albumin and sodium. This correlation demonstrates that the less the functional capacity of the liver, the lower the serum level of miR-122. There was a strong negative correlation between the miR-122 and the MELD score which provides further evidence of the importance of low levels of miR-122 in outlining the presence of decompensation and its complications.
An explanation for our findings lies in the diminishing number of hepatocytes as cirrhosis advances, thus the release of miRNAS-122 may be lower than in those patients with more abundant liver tissue.
Coagulation profile has been used as a surrogate marker for liver function, Serum bilirubin and albumin reflect the functional capacity of the liver as well. But these parameters may vary depending on nutritional status, the use of drugs or the occurrence of cholestasis and are biased by different half-life times. In the clinical setting this may be a source of confusion; they should not be viewed as the most reliable indicator of liver function. The serum level of miRNA-122 is not affected in a similar fashion and our results confirm the benefit of establishing miR-122 as an independent prognostic marker of liver compensation in clinical practice whether alone or as a supplement to the MELD or Child Pugh scores.
However, our study is not without limitation as we did not detect a cut-off value below which decompensation may occur so further evaluation to find a cut-off point is needed.
In conclusion, lower serum miR-122 levels are associated with ascites, spontaneous bacterial peritonitis and Hepatorenal syndrome. Therefore, serum miR-122 could be a new potential parameter for liver function and a prognostic marker in patients with liver cirrhosis. Further investigations are needed to validate our findings and so, introduce this new marker into clinical practice in the near future.
Core tip: Decompensated cirrhosis is a serious complication. Prognostic parameters guide clinicians to assess severity and survival. The Child Pugh and MELD score serve this purpose. The miRNA-122 is liver specific and was correlated to hepatic inflammation and cirrhosis. Our study aimed to prove that miR-122 could be of value as a prognostic marker of chronic liver decompensation. miR-122 correlated negatively with creatinine, urea and INR while it correlated positively with albumin, PC and sodium levels. It also showed a strong negative correlation with both the Child Pugh and MELD scores.
- Goldman L, Schafer AI (2012) Goldman's Cecil Medicine, 24th. Edition Link: https://goo.gl/4qwMh8
- Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, et al. (2003) A model to predict poor survival in patients undergoing Transjugular Intrahepatic Portosystemic shunts. Hepatology 11: 864-871. Link: https://goo.gl/Dz67EY
- Ginés P, Quintero E, Arroyo V, Teres J, Bruguera M, et al. (1987) Compensated cirrhosis natural history and prognostic factors. Hepatology 7: 122-128. Link: https://goo.gl/9oTGDH
- Gine`s P, Guevara M, Arroyo V, Rodes J (2003) Hepatorenal syndrome. 362: 1819–1827. Link: https://goo.gl/hHZP7D
- Garcia TG (2001) Current management of the complications of cirrhosis and portal hypertension: variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Gastroenterology 120:726. Link: https://goo.gl/VQv5dR
- Forman LM, Lucey MR (2001) Predicting the prognosis of chronic liver disease: an evolution from Child to MELD. Mayo End-stage Liver Disease. Hepatology 33: 473-475. Link: https://goo.gl/dLJCCe
- Attia KA, Ackoundou N'guessan KC, N'dri Yoman AT, Mahassadi AK, Messou E, et al. (2008) Child-Pugh-Turcott versus Meld score for predicting survival in a retrospective cohort of black African cirrhotic patients. World J Gastroenterol 14: 286-291. Link: https://goo.gl/7q2ieo
- Huo TI, Lin HC, Wu JC, Lee FY, Hou MC, et al. (2006) Proposal of a modified Child-Turcotte-Pugh scoring system and comparison with the model for end-stage liver disease for outcome prediction in patients with cirrhosis. Liver Transplant 12: 65-71. Link: https://goo.gl/g0v0qs
- Freeman RB Jr, Wiesner RH, Harper A, McDiarmid SV, Lake J, et al. (2002) The new liver allocation system: Moving toward evidence-based transplantation policy. Liver Transpl 8: 851–858. Link: https://goo.gl/wwfwYM
- Papatheodoridis GP, Cholongitas E, Dimitriadou E, Touloumi G, Sevastianos V, et al. (2005) MELD vs Child-Pugh and creatinine-modified Child-Pugh score for predicting survival in patients with decompensated cirrhosis. World J Gastroenterol 11: 3099-3104. Link:
- Chen KR, Rajewsky NR (2007) The evolution of gene regulation by transcription factors and microRNAs. Nature Reviews Genetics 8: 93-103. Link: https://goo.gl/vidZRv
- Lagos QM, Rauhut R, Yalcin A, Meyer J, Lendeckel W, et al. (2002) Identification of tissue-specific microRNAs from mouse. Curr Biol 12: 735-739. Link: https://goo.gl/UCWOYw
- Wang K, Zhang S, Weber J, Baxter D, Galas D (2010) Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res 38: 7248–7259. Link: https://goo.gl/zaoTVT
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, et al. (2008) Circulating microRNAs as stable blood based markers for cancer detection. Proc Natl Acad Sci USA 105: 10513–10518. Link: https://goo.gl/3iFZ4j
- Chang J, Nicolas E, Marks D, Lerro A, Buendia MA, et al. (2004) MiRNA-122 a mammalian liver-specific microRNA, CAT-1. RNA Biol 1:106. Link: https://goo.gl/jd6il4
- Shah N, Nelson JE, Kowdley KV (2013) microRNAs in liver disease: Bench to Bedside J. Clin. Exp.Hepatol 3:231-242. Link: https://goo.gl/Q8gDyQ
- Li J, Ghazwani M, Zhang Y, Lu J, Li J, et al. (2013) miR-122 regulates collagen production via targeting hepatic stellate cells and suppressing P4HA1 expression. J Hepatol 58: 522-528. Link: https://goo.gl/zrC4M6
- Zhang Y, Jia Y, Zheng R, Guo Y, Wang Y, et al. (2010) Plasma microRNA-122 as a biomarker for viral-, alcohol-, and chemical-related hepatic diseases. Clin Chem 56: 1830–1838. Link: https://goo.gl/wx4Isn
- Xu J, Wu C, Che X, Wang L, Yu D, et al. (2011) Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog 50: 136–142. Link: https://goo.gl/VA3Ow9
- Wang K, Zhang S, Mazola B, Troish P, Brightman A, et al. (2009) Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci USA 106: 4402–4407. Link: https://goo.gl/K6sAhs
- Starkey Lewis PJ, Dear J, Platt V, Simpson KJ, Craig DGN, et al. (2011) Circulating microRNAs as potential markers of human drug induced liver injury. Hepatology 5: 1767–1776. Link: https://goo.gl/74EiVV
- Gines P, Angeli P, Lenz K, Lenz K, Møller S, et al. (2010) EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 53: 397-417. Link: https://goo.gl/Kmov1u
- Bihrer V, Friedrivh-Rust M, Kronenberger B, Forestier N, Haupenthal J, et al. (2011) Serum miR-122 as a biomarker of necroinflammation in patients with chronic hepatitis C infection Am J Gastroenterol 106:1663- 1669. Link: https://goo.gl/fOYLUd
- Garcia Tsao G, Bosch J, Groszmann RJ, (2008) Portal hypertention and variceal bleeding –unresolved issues. Summary of an American Association for the study of liver diseases and European Association for the study of the liver single-topic conference Hepatology 47: 1764-1772. Link: https://goo.gl/jDAffe
- D’Amico G, Garcia Tsao G, Pagliaro L (2006) Natural history and prognostic indicators of survival in liver cirrhosis: systematic review of 118 studies J Hepatol 44: 217-231. Link: https://goo.gl/PKuLgs
- Sibae MR, Cappell MS (2011) Accuracy of MELD scores in predicting mortality in decompensated cirrhosis from variceal bleeding, hepatorenal syndrome, alcoholic hepatitis, or acute liver failure as well as mortality after non-transplant surgery or TIPS. Dig Dis Sci 56: 977-87. Link: https://goo.gl/LplQAg
- Yoo HY, Edwin D, Thuluvath PJ (2003) Relationship of the model for end-stage liver disease (MELD) scale to hepatic encephalopathy, as defined by electroencephalography and neuropsychometric testing, and ascites. Am J Gastroenterol 98: 1395-1399. Link: https://goo.gl/smDd2g
- Khot AA, Somani P, Rathi P, Amarapukar A (2013) Prognostic factors in acute-on-chronic liver failure: A prospective study from Indian J Gastroenterol 33: 119-124. Link: https://goo.gl/FtN1Tf
- Takaya H, Uemura M, Fujimura Y, Matsumoto M, Matsuyama T, et al. (2012) ADAMTS13 activity may predict the cumulative survival of patients with liver cirrhosis in comparison with the Child-Turcotte-Pugh score and the Model for End-Stage Liver Disease score. Hepatol Res 42: 459-472. Link: https://goo.gl/9IxKQU
- Licata A, Maida M, Bonaccorso A, Macaluso FS, Cappello M, et al. (2013) Clinical course and prognostic factors of hepatorenal syndrome: A retrospective single center cohort study. World J Hepatol 5: 685-691. Link: https://goo.gl/DhBRfO
- Zhang JY, Qin CY, Jia JD, Wang BE (2012) Serum sodium concentration profile for cirrhotic patients and its effect on the prognostic value of the MELD score. Zhonghua Gan Zang Bing Za Zhi. 20: 108-11. Link: https://goo.gl/Y0e8JC
- Janičko M, Veselíny E, Abraldes JG, Jarcuska P (2013) Serum sodium identifies patients with cirrhosis at high risk of hepatorenal syndrome. Z. gastero. J. 51: 628-34. Link: https://goo.gl/NnP9xg
- Shaikh S, Mal G, Khalid S, Baloch GH, Akbar Y (2010) Frequency of hyponatraemia and its influence on liver cirrhosis-related complications. J Pak Med Assoc 60: 116-20. Link: https://goo.gl/s9RldX
- Waidmann O, Köberle V, Brunner FM, Zeuzem S, Piiper A, et al. (2012) Serum microRNA-122 predicts survival in patients with liver cirrhosis. PLoS ONE 7: 45652. Link: https://goo.gl/YnHo3q
- Sumskiene J, Kupcinskas L, Pundzius J, Sumskas L (2005) Prognostic factors for short and long-term survival patients selected for liver transplantation 41: 39-46. Link: https://goo.gl/K68luO
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
