Archives of Hepatitis Research
The correlation between serum cytokine levels and liver cirrhosis in chronic hepatitis B patients: A meta-analysis
Gaofeng Cai, Zhengting Wang, Jun Yao, Kui Liu, Zhifang Wang, Yongdi Chen*, Huakun Lv*, Biao Zhou*, Chonggao Hu*
Cite this as
Cai G, Wang Z, Yao J, Liu K, Wang Z, et al. (2018) The correlation between serum cytokine levels and liver cirrhosis in chronic hepatitis B patients: A meta-analysis. Archives of Hepatitis Research 4(1): 006-010. DOI: 10.17352/ahr.000019Background: The harm of liver cirrhosis (LC) is serious, and the development of LC is primarily resulted from chronic hepatitis B virus (HBV) in high-risk such as China and Africa and chronic hepatitis C virus (HCV) in developed areas such as United States. Currently, there were 360 million chronic HBV-infected people on a global scale, and 30 million chronic hepatitis B (CHB) patients in China. In general, cytokines can regulate immune responses or contribute to deleterious tissue injury. However, the effects of these cytokines reported were controversial. Therefore, we executed a meta-analysis evaluating whether these cytokines can change the development risk of LC.
Methods: CHB patients were taken as participants, and studies were searched from Springer, Wiley, Chinese Medical Journal Database, PubMed, Elsevier, OVID, EBSCO, Mean difference (MD) with 95% confidence intervals (CI) were calculated by Review Manager 5.1.
Results: In this meta-analysis, 731cases and 1012 controls from 30 studies were analyzed. The pooled MD of the serum cytokines were transforming growth factor-β1 (TGF-β1): 25.86 (95% CI : 184.73-286.99)pg/ml, interleukin(IL)-6: 56.35 (95% CI : 19.00-93.70) pg/ml, IL-17:22.07(95% CI : 11.77-32.37) pg/ml, IL-10:-3.24 (95% CI : -4.11, -2.36) pg/ml, and interferon-γ (IFN-γ): 1.50(95% CI : -4.34-7.35) pg/ml, respectively.
Discussion: In CHB patients, elevated of serum levels for TGF-β, IL-6, and IL-17 can increase the risk of LC development, whereas elevated of serum levels for IL-10 decreased the risk. We suggest high-risk subjects with elevated of serum levels for these cytokines should be closely monitored and receive treatment timely for reducing the development of LC.
Abbreviations
HBV: Hepatitis B Virus; HBeAg: Hepatitis B e Antigen; HCV: Hepatitis C Virus; HCC: Hepatocellular Carcinoma; LC: Liver Cirrhosis; CHB: Chronic Hepatitis B; TGF-β: Transforming Growth Factor-β; IL: Interleukin; IFN-γ: Interferon-γ; CI: Confidence Intervals; MD: Mean Difference; CMJD: Chinese Medical Journal Database
Introduction
The harm of LC is serious. The 5 - year survival rate for compensated LC patients was 80% - 86%, and 14% - 30% for decompensated LC patients [1,2]. The development of LC is primarily resulted from chronic HBV in high-risk areas such as China and Africa and chronic HCV in developed areas such as the United States.
Currently, chronic HBV infection still is a serious threat to people. On a global scale, there were 360 million chronic infected population [3], and there were 30 million CHB patients in China [4]. The immune dysfunction and imbalance of immune regulation resulted in condition recurrent and worse of these CHB patients from one thing to another, and then resulted in hepatic fibrosis and even LC.
The basic reasons for the formation of liver fibrosis and LC is imbalance between synthesis and degradation of hepatic extracellular matrix.
In general, cytokines can regulate immune responses, and cytokines can mediate or contribute to deleterious tissue injury only under certain conditions.
Therefore, we research serum cytokines such as TGF-β, IL and so on.
TGF-β has regulatory functions in many biological processes, Such as extracellular matrix protein expression, cell proliferation, protein decomposition and expression of cell adhesion receptor. TGF-β also has regulatory functions in a variety of pathogenic process, such as inflammatory tissue repair, fibrosis and tumor. TGF-β in human body exist three isomers TGF-β1, TGF-β2, and TGF-β3 [5,6]. At present, only TGF-β1 function was recognized, in this study, only TGF-β1 was used as the research variables.
IL are small molecules active peptides produced by a set of many kinds of cells, regulating the body’s normal immune response, such as IL – 6, as inflammatory cytokines, have a strong chemotaxis and activated neutrophils role [7].
At present, there are effective treatments to improve these serum cytokine levels. In addition, these serum cytokines have been investigated as the possible risk factors for the development of LC by previous studies, and these serum cytokines also can be detected routinely in basic level hospitals, such as the studies for IL-6 [8-13], the studies for IL-17 [14-18], the studies for IL-10 [18-21], the studies for the studies for IFN-γ [22-26], and the studies for TGF-β [27-37]. However, the effects of these serum cytokines were controversial.
Random error can be reduced and test power can be increased by meta analyzing. In this study, we pooled MD with 95% CI for these serum cytokines in order to identify whether these cytokines changed the risk of LC development. And then to control the risk factors for high-risk groups and to decrease the development of LC.
Materials and Methods
Literature and search strategy
All articles were retrieved from Springer, OVID, PubMed, Elsevier, Chinese Medical Journal Database (CMJD), Wiley, EBSCO. Searches were done in search field “MeSH Terms”, and the search terms (“hepatitis B”) and (“liver cirrhosis”) and (“cytokines”) were used.
The present study was carried out following Meta-analysis in PRISMA guidelines [38].
Inclusion and exclusion criteria
Studies were included in this study when: [1], retrospective continuously or longitudinal study [2], original research published in English or in Chinese [3], Eligible research articles not captured by the research strategies detailed above were included by bibliography searches.
Studies were excluded from this study provided that: [1] the article reported simultaneously two or more kinds of hepatitis virus as the etiological agent [2], the article did not provide a workable value for the serum cytokines.
Data extraction
An assessment was executed by two independent reviewers based on a standardized data extraction form designed by our group so as to decide that an article was included or excluded. Data was extracted from each study by two separate reviewers.
Discrepancies between the decisions of the two reviewers were discussed for a settlement of all these discrepancies. Duplicate reports of the same articles were eliminated by checking.
Statistical analysis
The MD with 95% CI was used as the main outcomes to measure efficacy. The fixed-effect or random-effect model was used for executing Meta-analysis to pool the MD with 95% CI.
The statistical heterogeneity among studies was evaluated by Q test and I2 test. When P≤ 0.1 the random-effects model was operated, and when P>0.1 the fixed effects model was operated. Analyses were executed by the software Review Manager 5.1 (Cochrane Collaboration, https://www.cc-ims.net/RevMan/relnotes. htm). The MD wasn’t pooled when the number of MD of the serum cytokine marker were less than 5.
Results
Literature search
In this meta-analysis, thirty studies were eligible, and the flowchart of studies selected for inclusion in this meta-analysis was shown in figure 1.
Thirty eligible studies were identified and included in this meta-analysis.
Characteristics of the studies
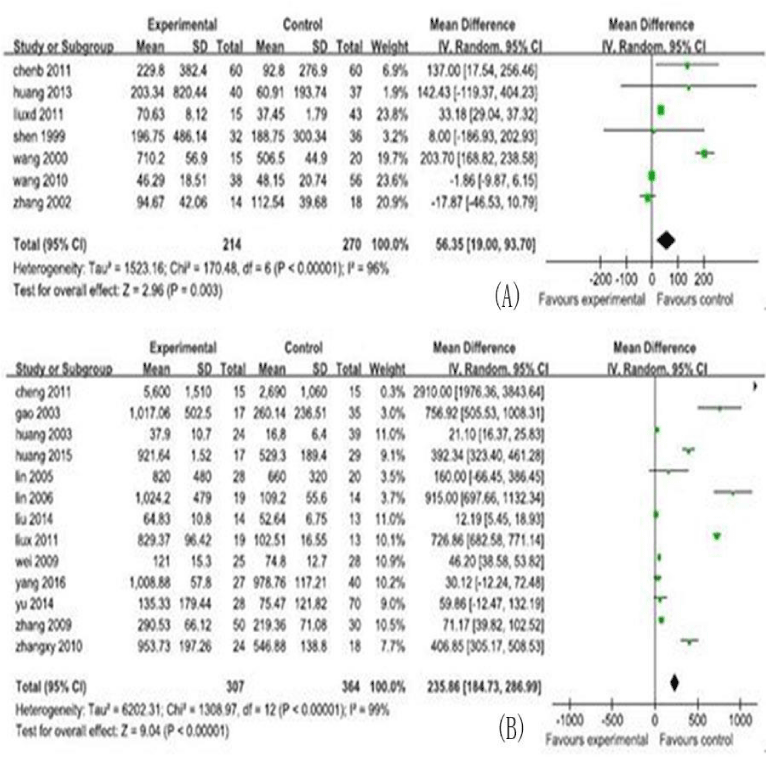
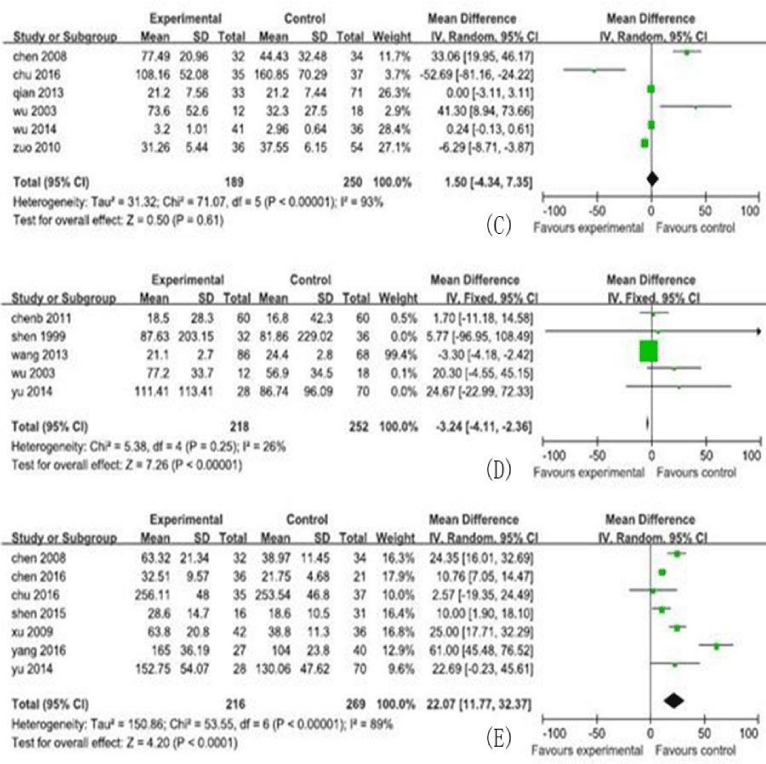
In this meta-analysis, 30 studies, and 731cases and 1012 controls from 30 included studies were analyzed, including the MD and their 95% CIs for serum cytokine markers, shown in figures 2,3. The characteristics of the studies, including number of reference, study region, study type, participants category for case/control, serum cytokine markers, sample size, male/ female and age (years), are shown in table 1.
Effects of related factors on the development of LC
In this analysis, the 5 serum cytokine markers analyzed were listed as follows: TGF-β1 (13 studies, 671 research objects), IFN-γ (6studies, 439 research objects), serum IL-6 (7studies, 484 research objects), serum IL-17 levels (7 studies, 485 research objects), and serum IL-10 levels (5 studies, 470 research objects), and the results are displayed in figures 2,3.
In this meta-analysis, 731cases and 1012 controls from 30 studies were analyzed. The pooled MD of the serum cytokines were transforming growth factor-β1 (TGF-β1): 25.86 (95% CI : 184.73-286.99)pg/ml, interleukin(IL)-6: 56.35 (95% CI : 19.00-93.70) pg/ml, IL-17:22.07(95% CI : 11.77-32.37) pg/ml, IL-10:-3.24 (95% CI : -4.11, -2.36) pg/ml, and interferon-β (IFN-γ):1.50(95% CI : -4.34-7.35) pg/ml, respectively.
The MD with 95% CI test showed that the variation of study-specific MD for serum levels for TGF-β, IFN-γ, IL-6, and IL-17 were statistically significant (p < 0.10), and then, the effects for these were pooled via operating the random-effect method, whereas the IL-10 for the fixed-effect method (p>0.10). The analysis results of serum cytokine levels were shown in figures 2,3.
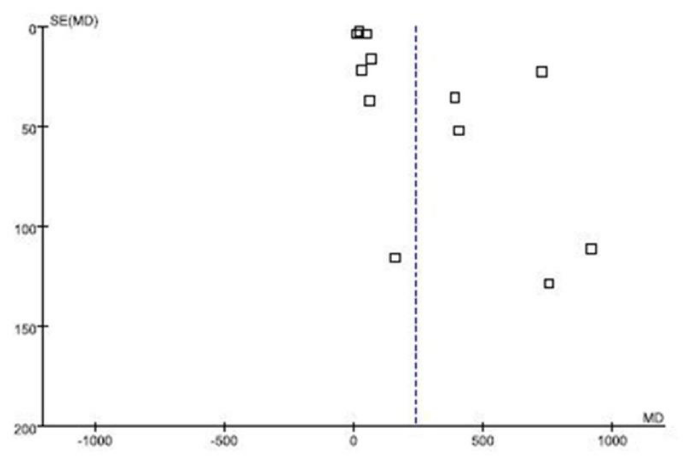
Publication bias
Articles published in the distribution is symmetrical and majority of the articles are in triangle of the funnel plot, and symmetrical axis is off center axis (MD=0) and is at the right side of the center axis. A funnel plot for published bias is shown in figure 4.
Discussion
Our meta-analysis demonstrated that, for CHB patients, elevated of serum levels for TGF-β, and IL-17 can significantly increase the risk of LC development. These finding was supported by the previous studies listed as follows: the studies for TGF-β [36,37,39], and the study for IL-17 [15], and these previous studies found that along with progressing of disease in liver, elevated of serum levels for these cytokines can goes up step by step. IL-6 can increase the risk of LC development. This finding was not consistent with the result of study by Zhou taking primary biliary cirrhosis as the research object [40].
Our meta-analysis demonstrated that, for CHB patients, elevated serum levels IL-10 can decrease the risk of LC development. These finding was confirmed by original studies [41]. In addition, IL-22, a member of the IL-10 family, that elevated of IL-22 levels can decrease the risk of LC development also was confirmed in animal and cytological experiments [42]. Whether IL-10 play a role in anti-fibrosis in CHB patients, and that needs further study and must meet the requirements of ethics at the same time.
Our meta-analysis also demonstrated that, for CHB patients, elevated serum levels IFN-γ didn’t change the risk of LC development. However, the study by Zhou found serum levels of IFN-γ in primary biliary cirrhosis patients was lower than that in CHB patients [40]. And that whether IFN-γ was connected with the risk of LC development remains to be further studied.
This study has two limitations: [1] in subgroup analysis, the sample size for IFN-γ and IL-10 was small [2], and only primary studies published in English or in Chinese were included. Two points above may be a slight impact on this study results.
Conclusion
In CHB patients, elevated of serum levels for TGF-β, IL-6, and IL-17 can increase the risk of LC development, whereas elevated of serum levels for IL-10 decreased the risk. We suggest high-risk subjects with elevated of serum levels for these cytokines should be closely monitored and receive treatment timely for reducing the development of LC.
We thanked Chu Zhang for checking the data in Zhejiang Chinese Medical University.
Funding
This work was supported by National Natural Science Foundation of China (grant numbers No. 31500751, 2015) and Ministry of Science and Technology supporting the National Scientific and Technological Major Project of China (grant numbers No. 2017ZX10105001–002, 2017).
Authors’ contributions
Study design: G.C., H.L, B.Z., Y.C., C.H. Statistical analysis and interpretation: H.L., Y.C. Manuscript preparation: H.L., Zhi.W, J.Y., G.C., K.L., Zhe.W. Critical review of manuscript: B.Z., Y.C., H.L., C.H. All authors read and approved the final manuscript.
- De Jongh FE, Janssen HL, De Man RA, Hop WC, Schalm SW, et al. (1992) Survival and prognostic indicators in hepatitis B surface antigen-positive cirrhosis of the liver. Gastroenterology 103: 1630-1635. Link: https://tinyurl.com/y9a6695u
- Fattovich G, Pantalena M, Zagni I, Realdi G, Schalm SW, et al. (2002) Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol 97: 2886-2895. Link: https://tinyurl.com/y9fym3ru
- Jury E (2003) International Consensus Conference on Hepatitis B. 13-14 September, 2002: Geneva, Switzerland. Consensus statement (short version). Journal of hepatology 38: 533-540. Link: https://tinyurl.com/y9x4m9fg
- XF Liang, YS Chen, XJ Wang, He X, Chen LJ, et al. (2005) Study on the sero-epidemiology of hepatitis B in Chinese population aged over 3-years old. Chinese Journal of Epidemiology 26: 4. Link: https://tinyurl.com/yatedhpv
- Tucker RF, Shipley GD, Moses HL, Holley RW (1984) Growth inhibitor from BSC-1 cells closely related to platelet type beta transforming growth factor. Science 226: 705-707. Link: https://tinyurl.com/y9gtcwpw
- Orsatti G, Hytiroglou P, Thung SN, Ishak KG, Paronetto F (1997) Lamellar fibrosis in the fibrolamellar variant of hepatocellular carcinoma: a role for transforming growth factor beta. Liver 17: 152-159. Link: https://tinyurl.com/y7tbbdmu
- P Wang, P Chang (2011) The clinical study of T cell subgroup, tumor necrosis factor - alpha, interleukin-6 and interleukin-8 in hepatitis B after hepatitis B. Chin J Postgrad Med 34: 70-72.
- YJ Wang, JP Gao (2010) Significance of the serum HBV-DNA and IL-1, IL-6 level in hepatitis B virus-related diseases. Fujian Medical Journal 32: 90-91.
- XD Liu (2011) Clinical significance and change of serum IL-6, T-lymphocyte subsets and HBV DNA in patients related to chronic hepatitis B. Shandong Medical Journal 51: 47-48.
- F Shen, ZT Zhou, DH Chen, M Chen (1999) Detection of serum Interleukins IL-6, IL-8 and IL-10 in patients with chronic hepatitis B. Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases 9: 31-32.
- JP Wang, XH Li, Y Zhu, et al. (2000) Detection of serum sIL-2R, IL-6, IL-8, TNF-α and lymphocytes subsets, mIL-2R in patients with chronic hepatitis B. World Chinese Journal of Digestology 8:763-766.
- SM Huang, YL Wu, L Chen, MH Wang (2013) Alteration and significance of Th17, IL 6 and TNF related cytokines of Patients with Chronic HBV infection. J Mod Lab Med 28: 74-76.
- Zhang W, Yue B, Wang GQ, SL Lu (2002) Serum and ascites levels of macrophage migration inhibitory factor, TNF-alpha and IL-6 in patients with chronic virus hepatitis B and hepatitis cirrhosis. Hepatobiliary Pancreat Dis Int 1: 577-580. Link: https://tinyurl.com/y9l4tjct
- M Chen, XY Guo, HX Jiang, et al. (2016) Expression of Th17 cells in patients with hepatitis B virus-associated liver disease and its significance. Chongqing Medicine 45: 592-597.
- HY Shen, MY Qian, SX Hang, XC Tong (2015) Expression of T helper cell 17 in peripheral blood of patients with different degrees of HBV infection and its correlation with HBV-DNA and ALT. Guangxi Medical Journal 37: 1732-1736.
- Y Xu, SJ Chen (2009) Expression and significance of Interleukin—17 in chronic HBV related liver diseases. Shandong University 5.
- C Yang, F Cui, LM Chen, XY Gong, B Qin (2016) Correlation between Th17 and nTreg cell frequencies and the stages of progression in chronic hepatitis B. Mol Med Rep 13: 853-859. Link: https://tinyurl.com/y92otpfj
- X Yu, R Guo, D Ming, M Su, C Lin, Y Deng, et al. (2014) Ratios of regulatory T cells/T-helper 17 cells and transforming growth factor-β1/interleukin-17 to be associated with the development of hepatitis B virus-associated liver cirrhosis. J Gastroenterol Hepatol 29: 1065-1072. Link: https://tinyurl.com/y8vg34tx
- SQ Wang, JY Wang, YQ Chen, J Man (2013) The Clinical significance of levels of serum IL-18 and IL-10 in patients with posthepatitic cirrhosis. Journal of Qiqihar University of Medicine 34: 1894-1895.
- JL Wu, JZ Wu, GH Lou, JN Jiang (2003) The influence of cytokines on the occurrence of HBVDNA BCP mutation, state of an illness and the replication of HBV in patients with chronic hepatitis B. Journal of Guangxi Medical University 20: 344-346.
- B Chen, QY Liu, L Sun, J Ye (2011) A preliminary study on the relationship between anxiety, depression and cytokines in patients with chronic hepatitis B and posthepatitic cirrhosis. Chinese Hepatology 16: 237-239.
- LX Qian, HY Zhang, YZ Wang (2013) Clinical Application of Th1/Th2 Cytokines in Chronic Hepatitis B, Liver Cirrhosis and HCC Patients Liver Cirrhosis and HCC Patients. Journal of Radioimmunology 26: 59-60.
- YL Wu, SM Huang, L Chen, MH Wang (2014) Expression and the clinical value of helper T lymphocyte in the patients with chronic HBV infection. China Journal of Modern Medicine 24: 46-49.
- SJ Chen, WJ Du, ZZ Xing, Y Xu, KS Xu (2008) Expression of TH17 in the patients with chronic HBV infection and its’ relationship with liver fibrosis. Shandong Medical Journal 48: 21-23.
- WZ Zuo, LH Shen, CM Xu, kM Maili, Qun Tian, Rong Ji (2010) The relationship of the XCL1,IFN-γ and T cells in hepatitis B cirrhosis. Chinese Journal of Gastroenterology and Hepatology 19: 811-814.
- YL Chu, HL Gu, J Lan, SM Chen, J Tang, WL Zhang (2016) Biomarkers in peripheral blood T——lymphocyte subsets of patients with chronic Hepatitis B and its advanced liver diseases. Practical Preventive Medicine 23: 873-876.
- XD Liu (2011) Quantitative analysis of Serum Interleukin-18、Transforming Growth Factor beta1, ALT, TBIL and HBV-DNA levels in Chronic Hepatitis B. Shandong Medical Journal (51)23: 66-67.
- L Wei, H Chen (2009) Correlation between Toll-like receptor 4 on Peripheral blood mononuclear cells(PBMC) and serum TGF-β in patients with chronic hepatitis B virus infection. Lanzhou University 6.
- JN Liu, XL Zhu (2014) The significance and Relationship among the Matrix Metalloproteinase-2, Transforming Growth Factor-β1 and Cystatin C in patients with chronic hepatitis B and Cirrhosis. Dalian Medical University 4.
- CM Zhang, ZL Fan, XH Wang (2009) Changes and significances of serum and urinary transforming growth factor BETA-1 in patients with hepatitis b virus-related chronic liver disease. Journal of Qinghai Medical College 30: 43-45.
- Y Cheng, XZ Jin, C Ye, MH Zheng (2011) Association between CD4+ CD25+ Regulatory T Cells in Peripheral Blood of Patients with HBV DNA Resolved Chronic Hepatitis B and Different Chronic Liver Process After Treated with Nas. Journal of Medical Research 40: 50-54.
- YL Gao, DX Lin, WM Huang, Y He, P Zhang, QL Ye (2003) Relationship between Serum TGF-β1 Level, and Liver Function or Liver Fibrosis in Patients with Hepatitis B. Journal of Fujian Medical University 37: 247-249.
- XY Lin, YY Wu, ZH Tao, XD Chen, GX Shen, JM Wu (2005) Study on serum level of Fas and TGF-β1 in patients with HBV related cirrhosis of liver and hepatocellular carcinoma. Chinese Journal of Laboratory Diagnosis 9: 102-104.
- SM Huang, YL Wu (2003) The effect of serum TGF-1 of patients with chronic virus hepatitis B in liver fibrosis formation. Journal of Mudanjiang Medical College 20: 10-12.
- XY Zhang, Y Yang, HP Sheng, YF Lai (2010) Relationship between serum levels of transforming growth factor β1 and hepatic fibrosis in chronic hepatitis B patients. Shandong Medical Journal 50: 4-6. Link: https://tinyurl.com/ycxsqhgo
- GD Huang, YQ Xie, BX Yang, F Huang (2015) Study of serum TGF-β_1 levels. in patients with chronic hepatitis B virus infection and its correlation with hepatic fibrosis. Hainan Medical Journal 26: 1890-1891.
- DX Lin, YL Gao, WM Huang, P Zhang (2006) Stepwise Discriminate Analysis of Serological Detection of the Viral Hepatitis B. Journal of Fujian Medical University 40: 44-47.
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6: e1000097. Link: https://tinyurl.com/yabycgub
- Y Kang, J Shang, XL Li, YX Yang (2006) Detection of Serum Transforming Growth Factor β-1 and Leptin in Chronic Hepatitis B and Hepatitis Cirrhosis of Liver Patients. Chinese Journal of Clinical Gastroenterology 18: 270-274.
- YH Zhou, HX Ma, GY Cao, GH Rong, YS Xiong, RQ Zhong (2006) Expression of TLR and SOCS in peripheral blood mononuclear cells of patients with primary biliary cirrhosis. Journal of Yangtze University (Natural Science Edition) 3: 219-225.
- WH Pan, PA Zhu (2013) Clinical significance and detection of serum CHE, HA, IL-10 and IL-2 in hepatitis B patients. Journal of Hainan Medical University 19: 791-793. Link: https://tinyurl.com/yclcsnl6
- Kong X, Feng D, H Wang, Hong F, Bertola A, et al. (2012) Interleukin一22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology 56: 1150-1159. Link: https://tinyurl.com/y78hlx5y
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
