Archives of Hepatitis Research
Experience with Hepatitis B Viral load Testing in Jharkhand
Sana Irfan1*, Mahua Das Gupta2, NP Sahu3, Zulfiquar Ali Bhuttoo4 and Poonam Kumari4
2Department of Microbiology, Bhagirathi Neotia Mother and Child Care Centre, Newtown, Kolkatta, India
3Department of Microbiology, Narayan Medical College and Hospital, Sasaram, Bihar, India
4Department of Health and Research Viral Research & Diagnostic Laboratory, Department of Microbiology, Rajendra Institute of Medical Sciences, Ranchi, Jharkhand, India
Cite this as
Irfan S, Gupta MD, Sahu NP, Bhuttoo ZA, Kumari P (2019) Experience with Hepatitis B Viral load Testing in Jharkhand. Arch Hepat Res 5(1): 009-016. DOI: 10.17352/ahr.000021Background: Quantification of the viral burden is an important laboratory tool in the management of hepatitis B virus (HBV)-infected patients. However, widespread use of assays is still hampered due to the high cost. Treatment reduces viral load to undetectable levels. HBV infected patients tend to have high HBV DNA levels, and severe liver disease.
Objectives: This study was carried out to determine the pattern of HBV viral load levels of patients in Jharkhand.
Method: Variables included socio demographics like age, sex, occupation, history of vaccination, surgery, IVDU.etc. The COBAS Amplicor automated Analyzer (PCR based) was used to assay the virus quantitatively.
Results: 366 number of patients were tested from 2013 to 2015. HBV viral titre ranged between. <6 IU/ml to>1.1 × 108.
Conclusion: There was a high occurrence of viral titre as well as suspected core mutants in the population studied. Viral load is a risk factor for cirrhosis and hepatocellular carcinoma. A policy earmarked to combat this virus in Jharkhand is hereby solicited.
Introduction
It was estimated that 350 million individuals with chronic HBV infection had 15%-25% risk or chances of dying due to HBV-related advanced liver diseases, which includes Hepatocellular carcinoma (HCC) and decompensated cirrhosis [1]. HCC is the fifth most common global health problem, and the third leading cause of cancer-related death [2]. Regions where HBV is hyperendemic like those in East Asia and sub-Saharan most of the carriers of chronic hepatitis B virus develop HCC [3]. Each year more than 500000 new HCC cases with an age-adjusted incidence of 5.5-14.9 per 100000 populations worldwide are diagnosed [2]. It has been reported that chronic viral infection HBV (50%-55%) and HCV; (25%-30%) is the cause of 75%-80% of global HCC patients. [4]. Patients with chronic HBV infection have risk of greater than 100-fold of developing HCC as compared with non-carriers [5]. Due to HBV infection high related morbidity and mortality the global disease burden is important .Persistent suppression of HBV DNA is very important to prevent the development of HCC in cirrhosis patients [6]. The success rate is 50%-70% in reducing viral load to undetectable levels when treated with the interferon group. The progression of chronic hepatitis B to HCC is a multistage process. The nonviral risk factors may include aflatoxin exposure, alcohol drinking, age and host susceptibility factors [7-9]. In order to study the dynamics of HBV infection markers and their impact on disease progression risk a long-term follow-up study with repeated measurements of key risk factors would be the best way [10]. The natural course of chronic HBV infection is highly variable from patient to patient depending on time lag of infection and age of the infected patient. Early life or perinatal infection is characterized by a period of “immune tolerance” where the host coexists with the virus without apparent injury to the host. The serum HBV DNA levels are persistently high during this period. The patients can then progress through an immune active phase or immune clearance phase followed by immune tolerance phase where hepatitis becomes active, the host immune system tries to clear infected hepatocytes. During this phase there is hepatic inflammation, serum alanine aminotransferase (ALT) elevations, and good level reduction of the circulating HBV DNA levels. The immune clearance phase is highly variable in duration and frequency, but a prolonged phase or recurrent episodes of acute liver inflammation may result in repeated cycles of injury and regeneration resulting in necroinflammation/fibrosis and an increased risk of progression to cirrhosis and HCC [11].
The recent studies indicate that HBV-DNA levels strongly predict development of HBV-related cirrhosis and HCC in Asian cohorts [12–14]. There is limited information on HBV-DNA detection and quantification in developing Asian countries because it is still a costly enterprise which keeps the test out of reach of the average economic status citizen. However, the HBV DNA test, essential for hepatitis B management, introduced in (Rajendra Institute of Medical Sciences) RIMS Ranchi had made the test reach to every class of people due to low cost of testing and free testing under government provision. The purpose of this study was to obtain the pattern of HBV viral load levels of people in different age, sex and predicting the patient with the risk of cirrhosis and HCC in patients infected with HBV.
Materials and Methods
Study area
The study was carried out at Virus Research Diagnostic Laboratory, Department Of Microbiology, Rajendra Institute of Medical Sciences, Ranchi, Jharkhand from 2013-2015. Jharkhand, a state in eastern India .Most of the patients attending the hospital came from the city and surrounding districts.
Study population
Subjects included patients attending inpatient and outpatient facility at Rajendra Institute of Medical Sciences, Ranchi; Jharkhand for whom HBsAg detection was sought as diagnostic and screening purpose for a period of two year (2013-2015).A total of 366 patients diagnosed positive for HBsAg came for viral load estimation. All patients belong to the lower and middle class socio economic status.
Ethical issues
The Institute Ethics Committee had granted permission for carrying out this research work.
Data collection
A questionnaire was framed to obtain demographic details of all patients who tested positive for HBsAg which include hospital administration number, age, sex ,district, occupation, history of jaundice ,family history of Hepatitis B positivity, alcohol drinking habit, Intra venous drug abuse etc
Sample collection and processing
Venous blood samples were collected aseptically. Approximately 5 ml whole blood was collected from each patient in a labelled sterile plain vacutainer using a disposable sterile needle and syringe. Blood was allowed to clot for 30 minutes followed by centrifugation at 10000 RCF for 15 minutes. Serum was separated and aliquoted .Samples was stored at -80 °C for long term storage.
Diagnostic criteria
The diagnostic parameters were clearly outlined as per the standard procedures and WHO guidelines. Care was taken to avoid any bias opinion or definition as per the literature. The details of the definition followed are as follows.
Acute hepatitis
Acute Hepatitis is typically diagnosed by the presence of HBc (hepatitis B core) IgM. Patients have subclinical or anicteric hepatitis. It is characterised by nonspecific symptoms of malaise, poor appetite, nausea and pain in the abdomen right upper quadrant. During the icteric phase, fatigue and anorexia usually worsen. Jaundice can last from few days to several months, but usually 2–3 weeks. Itching in body and pale colored stools may occur. The convalescent phase begins with the resolution of jaundice. The physical signs may include variable degrees of jaundice, mild and slightly tender hepatomegaly and mild enlargement of spleen and lymph nodes. Few cases are of fulminant hepatitis. The typical presentation of severe reactivation (HBc IgM positive) is a short onset of jaundice and very high ALT (alanine transferase) level, sometimes preceded by prodromal constitutional symptoms, in a patient with chronic hepatitis B. If signs of chronic liver disease are present, the diagnosis could be easy of reactivation. ALT level deranged from the normal range.
Chronic hepatitis
The infection if extends more than six months is called chronic infection and is diagnosed by negative HBc IgM and positive HBc Total antibody (Ab). Three phases are identified: an immune tolerant phase with high HBV DNA and normal alanine aminotransferase (ALT) levels associated with minimal liver disease; an immune active phase with high HBV DNA and elevated ALT levels with active liver inflammation; and an inactive phase with HBV DNA levels < 2000 IU/mL and normal ALT levels with minimal inflammation and fibrosis on liver biopsy. Affected persons can move progressively from one phase to the next and may revert backward.
Exclusion criteria
The progression of chronic hepatitis B to HCC due to nonviral risk factors includes aflatoxin exposure, alcohol drinking, and host susceptibility factors. [8,9]. Such factors exposed patients were excluded from the study .Only naive detected cases were included in the study which were not treated for HBV infection or were recently diagnosed.
Laboratory investigation
HBsAg positive patients were tested for Hepatitis B surface antibody (HBs Ab) , HBc total Ab, Hepatitis B envelope antigen (HBeAg),antibody {anti} HBeAg and HBcIgM, by chemiluminescence ARCHITECT i1000SR instrument and finally viral load was detected.
HBV DNA viral load assay
Samples were assayed for the quantity of hepatitis B virus by the HBV Cobas Taqman 48 Real time PCR machine. The DNA was extracted by High Pure System Viral Nucleic Acid Kit and the real time detection was done by COBAS® TaqMan® HBV Test. The HBV viral load results are expressed in DNA IU/ml value by the automated analyzer. Specimen results are interpreted as follows, table 1 :
Results
(Tables 2-8, and figure 1)
Discussion
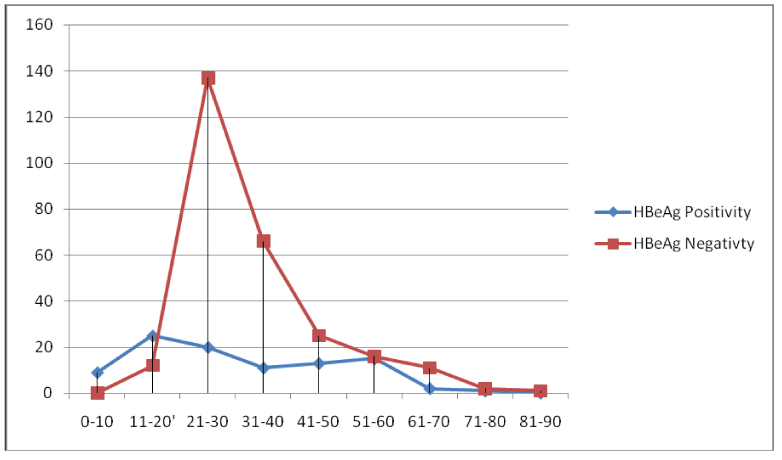
The natural road map of hepatitis B virus infection and its outcome is determined by the degree of virus replication and host immune response both. Infection with HBV is limited in developed countries but it is still a serious public health issue in developing countries like India [15]. In our study we had examined the viral load pattern of the patients attending the tertiary care centre, Rajendra Institute of Medical Sciences, Ranchi (RIMS), Jharkhand. Samples were received from different districts of the state. In this study also, more males requested for the test compared to females. The observation is same as observed in previous studies [16,3]. The patients included 32.24% (118) numbers of females and 67.75 % (248) numbers of males thus the number of male participant was higher. The distribution of HBsAg positivity among the patients shows increasing trend from the lower age group 0-10 to 21-30 and then a decreasing trend from 31-40 to 81-90 age group. The HBsAg positivity frequency in different age groups shows that lower and higher age groups are less exposed to risk factors as compared to the intermediate age groups. Thus we conclude that positivity is directly proportional to the exposure. The highest positivity is found between age group 21-30 (42.89%) which is the most sexually active age group. The result is the same as concluded in the previous study (Table 2) (16). Total 366 patients were tested for HBV DNA quantification in which 5.73% (21) cases were of acute and 94.26% (345) cases were chronic. Of all 15.30% (56) symptomatic and 84.69% (310) were asymptomatic cases. In the symptomatic cases 19.64% (11) were acute while 80.35% (45) were chronic cases. The 72.72% (8) symptomatic acute cases were HBeAg negative and 27.27% (3) were HBeAg positive. [Table 3]. The difference of HBeAg positivity between the 0-20 yrs and >20 years had been found significant.(df=1 χ2=61.8 p=0.0001) [Figure 1].
HBeAg and HBV-DNA detection: Role in risk of cirrhosis and HCC
In HBeAg positive cases the number of patients increases gradually from lowest to highest range of viral load .The maximum number (73) of patients were found in the range 107 IU/ml. None of the patients were found in the range TND and < 6. The higher HBeAg positive rate is usually associated with an increased risk of HCC since HBeAg positive is an indicator of active replication of HBV [17]. In contrast in HBeAg negative cases we could see the maximum number of patients (136) lies 6-1999 IU/ml viral load range, 18 patients had DNA /target not detected and 20 patients in < 6 IU/ml (Table 4) range which clearly indicates that HBeAg negatives cases basically depicts no active replication but there is unique thing though the replication is not active but still chronic HBeAg negative cases are found in higher range viral load too which will be discussed further. HBeAg is an alternative protein coded by four overlapping open reading frames of HBV genome designated as core region sequence and translation starts from precore gene containing a leader sequence which directs the HBeAg to the secretory pathway. HBeAg positivity is the sign of active HBV replication thus the presence of HBeAg was often used as a criterion for treatment before the introduction of HBV DNA examination. HBeAg has an immunomodulatory role [18]. A significant difference had been found between the chronic HBeAg positive/negative and symptomatic/ asymptomatic case (χ2=5.45, df=1, p=0.019). (Table 3) Thus in chronic cases the HBeAg positivity and HBeAg negativity affects the chance of the patient to be symptomatic and asymptomatic. In predicting the effect of HBeAg positivity on risk of HCC remains debatable. Multiple episodes of seroreversion from HBeAg-negative to HBeAg-positive was seen to increase the risk significantly for hepatocellular carcinoma in the study of Alaskan natives [19]. These studies suggest during the immune clearance phase high levels of circulating HBV DNA may be associated with the different grades of liver injury, which in turn may lead to the liver cirrhosis in these patients. Significantly higher HBeAg prevalence was reported among HBsAg positive HCC patients than that among matched HBsAg positive controls in other studies [20-22]. Significant increased in HCC risk was reported in a large prospective study conducted in Taiwan, in HBeAg positive patients [23]. Thus results from several prospective studies indicated that HBeAg was most likely a marker of active HBV replication correlated with an increased HCC risk.
HBeAg negative and HBV-DNA detection: Role in risk of cirrhosis and HCC
CHB can be broadly divided into two major forms—namely, HBeAg positive and HBeAg negative. CHB HBeAg negative is also referred to as anti-HBe-positive and precore mutant hepatitis. HBeAg-negative CHB patients are naturally occurring mutant form of HBV that does not produce HBeAg due to mutation in the precore or core promoter region of the HBV genome. The most frequent precore mutation is change at nucleotide 1896 (G1896A), G-A resulting in loss of HBeAg synthesis due to creation of stop codon due to nucleotide change. The most common mutation is in core promoter which involves substitution of two-nucleotide at nucleotides 1762 and 1764 (24). Heterogeneous group HBeAg-negative carriers generally have low viral DNA levels (< 104 copies/ml or 2000IU/ml), relatively normal levels of alanine aminotransferase (ALT) and a fair prognosis. Mutation in HBV pre-core is thought to be a mechanism of the virus to avoid the host immune response considering HBeAg is the target antigen which causes failure of immune response to destroy the virus. Many studies showed that there are relative anti-viral resistance in patients with pre-core mutation. [25-26]. The presence of pre-core mutation is usually correlated to chronic and severe liver disease. After meta analysis from 85 case control studies it was found that pre-core HBV mutation possibly contributes to hepatocelular carcinoma risk [27]. In some countries, pre-core mutation were reported in fulminant hepatitis cases; however, some reports in other countries showed that pre-core mutant was not related to fulminant hepatitis [28-29]. However about 15–20% of the carriers in Asia, the Middle East, Mediterranean basin and southern Europe had raised ALT and high viral DNA (104 copies/ml). In CHB HBeAg-negative emerges during a typical HBV infection with the wild-type HBV virus, and is selected during the HBeAg seroconversion (immune clearance phase). In a study done in Iran mutations in the precore region was found in 46% of the patients, among which 82.6% were HBeAb positive. Mean viral load in these patients with the precore mutation was high, 26% had viral load above 105 copy/ml and ALT was in the upper normal range. These studies suggest high viral loads in patients with the precore mutation [30-32].
In Table 4 HBeAg negative patients formed major (73.77%) part of the total population undergone viral load testing. The chronic HBeAg negative population with viral load ≥2000 IU/ml formed 37.5% of the population thus predicted to be core mutant. Thus from above discussion it is concluded the patients with viral load ≥ 104 copies/ml or ≥ 20000 IU/ml are predicted to be core mutant as they show higher DNA concentration which is the sign of core mutation. After statistical analysis a significant difference was obtained in the frequency of the patients in HBeAg positive and HBeAg negative group in the viral load range of 0-1999 IU/ml and ≥ 2000 IU/ml. (df=1, χ2=88.96 p=0.000).This indicates despite replication predicted in HBeAg negative core mutants they do not have that rate of replication activity as that in HBeAg positive groups. The arrow direction in Table 4 depicts the increasing order of frequency of patients for both chronic groups
HBV-DNA levels and risk of HCC and cirrhosis
According to a Taiwanese study the risk of HCC is closely associated with HBV [33]. This association was found to be independent of other risk factors, and there is dose-response relationship. It was also found that HBV DNA levels were elevated in patients at highest risk of liver cancer constantly. [34]. In a study high risk of hepatocellular carcinoma was observed among the population because of the high viral load. [35]. Increased risk for cirrhosis and for HCC with increasing level of HBV DNA was found in a large number chronic HBsAg carriers in Taiwan followed up for a long period [36-37]. Several studies have suggested an increased risk of HCC with high HBV DNA level > 106 copies/ml, 104-107 copies/ml or > 105 copies/ml [38]. In a study conducted in China the reports suggested that the patients with HBV DNA level higher than 104 IU/ ml had more risk to be diagnosed as HCC, which was similar to the previous prospective reports [37-41]. In one more study conducted in Gambia, West Africa it was found that high HBV-DNA levels are strongly associated with the serious squeal of HBV infection. As most studies suggest risk for cirrhosis and for HCC notably increases at HBV-DNA levels ≥10,000 copies/ml, low-level viremia were also linked with significant risk for HCC [42-44]. The commonly used quantitative units of HBV DNA level are IU/ml and copies/ml. In the current WHO HBV standard and consensus, one IU is approximately equivalent to five genome equivalents (copies). Chen and colleagues reported risk for cirrhosis and HCC outcomes achieving statistical significance at ≥10 000 copies/mL [23,45]. The viral load of 2000 IU/ml is approximately equal to 104 copies/ ml [46]. A hospital-based cohort study in Honkong including 1006 patients with a follow-up period of more than 7years showed similar results as the previous mentioned studies that serum HBV DNA levels are significantly associated with subsequent development of HCC in a dose-response relationship [47]. Risk of liver complications begins to increase significantly at the level of ≥104 copies/ml, adjusted hazard ratio (95% CI) for HCC risk was 2.3 (1.1–4.9) [48], and for cirrhosis this was 2.5 (1.6–3.8) was suggested from the data of R.E.V.E.A.L.-HBV study. Since HBV viral load is an independent predictor of risk of HCC, this points suggest the importance of continuous clinical monitoring and antiviral treatment for patients with high HBV viral load ≥10, 000 copies/ml or ≥2000IU/ml [49].
In the Table 4 we could see in all the chronic cases there are 51.88 % (192) patient with viral load ≥ 2000 IU/ml who attended for viral load determination to the centre irrespective of HBeAg status. Thus these patients at the risk of cirrhosis and HCC while 47.56% (174) are not at the risk for the same. Thus more than half the affected population are at the risk of cirrhosis thus it is recommended that the patients should be encouraged to go for the viral load determination as we could see the patients involved in the study is less as compared to diagnosed [16], so that the untested population could get the treatment at the right time. Probably the patient coming to centre for the viral load testing is less due to high cost of the test thus the government should take initiatives to provide free or cheap testing to the population of Jharkhand
HBV DNA in acute HBV infection
In acute HBeAg positive patients (Table 5) some cases were reported with USG findings of chronic liver disease with gross ascitis. These two cases seem to be the case of reactivation of chronic HBV infection. They were reported with jaundice as well USG finding of CLD with gross ascitis, splenomegaly and hepatomegaly. Upon exposure to hepatitis B virus (HBV), individuals develop acute self-limited hepatitis with a vigorous and broad immune response. However, acute HBV is rarely detected. It requires a high index of suspicion in the case of reactivation of HBV most often presents as acute viral hepatitis B (AVH-B) and clinically, it is difficult to differentiate AVH-B from reactivation of chronic hepatitis B (CHB). HBV related liver disease should be investigated by liver biopsy, endoscopy and/or imaging when patient has high HBV DNA (>2 ×104 IU/ml) level. The severity of acute infection and the stage of underlying chronic liver disease decide the degree of liver failure. Common causes of reactivation are mutations in the HBV genome, immunosuppressive therapy and viral or drug induced injury. Antiviral therapy is not required in the case AVH-B as in most of the cases such infections resolve spontaneously. However, in patients with CHB reactivation the use of a potent oral nucleoside (tide) analogue is necessary as soon as possible. In patients who develop liver failure secondary to severe acute exacerbation are considered for liver transplantation. If this is not feasible, supportive therapy with the addition of granulocyte colony stimulating factor (GCSF) therapy could be beneficial .Studies from China and Honkong reported chronic hepatitis B patients who developed severe reactivation (50-51). Usually acute cases are not tested as they do not require treatment as in most of the cases it gets resolved. Few acute cases reported above in the study were tested for viral load by mistake. Acute HBeAg negative cases were probably those cases which about to turn negative HBcIgM or chronic with rise in HbcIgG but due to very sensitive detection method of chemilluminiscence very low HbcIgM was detected.
HBV DNA in HBV-HIV co infected cases
In the current study the HIV-HBV coinfected cases viral load was also observed..The maximum prevalence was found among 31-40 years. The highest frequency of HIV HBV co-infected patients is different from the pattern obtained previously in HBsAg positivity study (21-30 years) (Table 6). In the Table 7 it was observed that maximum number of patients are found in higher viral load group both in HBeAg positive and HBeAg negative group. The viral load range group ≥2000 IU/ml in HBeAg negative are active HBeAg groups which are actively replicating but appears HBeAg negative due to basal and core mutation. Presence of HBeAg positive cases in HIV-HBV co-infected patient was significant (p=0.001 df=1 χ2=18.320). The risk of developing chronic HBV increases in the presence of HIV prior to HBV infection. [52], In our study we find the all co-infected patients were chronic. In Table 7 we find most (18) of the cases (62.06%)] are HBeAg positive and 10 (35.71%) HBeAg negative. All were asymptomatic diagnosed during screening only 1 (3.44%) was chronic symptomatic Anti HBe/ HBeAg -ve/-ve (Table 8). Higher prevalence of HBeAg-positive disease in HBV-HIV co-infected cases are found since the coinfection reduces the rate of spontaneous HBeAg seroconversion [53]. It is observed that maximum number of patients are found in higher viral load group both in HBeAg positive and HBeAg negative group leading to liver damage more rapidly [53]. Such coinfected patients have a poor response to interferon therapy and increased risk of liver-related complications and death. A major reduction in the incidence of opportunistic infections due to introduction of HAART in 1996 which led to the emergence of liver disease as one of the emerging causes of death in patients with HIV. In a retrospective review in 1998 – 1999 in a cohort of HIV-infected individuals, 50% of deaths happened due to end-stage liver disease when compared with deaths in 1991 and 1996 was less than 14% [54]. A large cohort study showed that death due to liver-related complications in HBV-HIV co-infected was 14.2 per 1000 person-years, HBV monoinfection was less than 14% of and 1.7 per 1000 person-years for HIV alone [55]. In liver-related death, with an adjusted relative risk of 3.73 active HBV was an independent predictor. The effect of HBV on HIV disease progression is not clear. A hypothetical assumption was done that HBV enhance HIV transcription that might enhance HIV replication leading to more rapid reduction in CD4 cell counts in HBV-HIV co-infected patients but there is little evidence to describe this [56,57].
Conclusion
It was identified that the productive viral replication also commonly occurs in the absence of HBeAg, Our findings extend the utility of HBV-DNA measurements in patients with 10 000 copies/mL of HBV DNA where an important decision in the antiviral treatment is taken as at this threshold cirrhosis or HCC risk in HBV-infected persons is predicted .It was concluded that high levels of circulating viral DNA trigger the immune responses that lead to liver injury in human beings. Accumulation of liver injury over time then manifests as end-organ damage. Thus one time point measurement of HBV DNA may not be insufficient to realize the grade of liver damage rather serial examination of viral load in case of chronic infection. This helps differentiate immune-tolerant individuals with quiescent disease from subjects with more active immune responses and persistent liver injury. The goals of HBV therapy are to stop or reverse progression of liver related complications by suppressing HBV replication.
We also conclude that clinical studies are required to obtain the threshold HBV DNA level for the initiation of anti- HBV treatment in HIV-HBV co-infected patients. It is also required to know how to optimize anti-HBV therapy in HIV-HBV co-infected persons, like combinations of drugs. Finally, different patterns of drugs resistance that had emerged in this population and how to minimize the antiviral drug resistance from long-term anti-HBV therapy.
The authors want to extend their heartfelt thanks to the Indian Council of Medical Research, New Delhi and RIMS, Ranchi for funding the study. The cooperation of Dr Seema Jha and Mr S. B. Singh (for his support in statistical work) is also acknowledged.
- Iloeje UH, Yang HI, Su J, Jen CL, You SL, et al. (2006) Predicting liver cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 130: 678-686. Link: https://tinyurl.com/y5wsjfjr
- Su Jong Yu, Yoon Jun Kim (2014) Hepatitis B Viral load affects prognosis of hepatocellular Carcinoma. World J gastroenterology 20: 12039-12044. Link: https://tinyurl.com/y3bqsjec
- Chen C, Yang H, Su J (2006) Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 295: 65–73. Link: https://tinyurl.com/y6n6qd34
- Yang HI, Lu SN, Liaw YF, You SL, Sun CA, et al. (2002) Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med 347: 168-174. Link: https://tinyurl.com/yyljyb6m
- Lee WM (1997) Hepatitis B virus infection. N Engl J Med 337: 1733-1745. Link: https://tinyurl.com/y6svtn7l
- Asim M, Malik A, Sarma MP, Polipalli SK, Begum N, et al. (2010) Hepatitis B virus BCP, Precore/core, X gene mutations/genotypes and the risk of hepatocellular carcinoma in India. J Med Virol 82: 1115-1125. Link: https://tinyurl.com/y3sejczj
- Apurva AM, Jordan JF (2007) Viral hepatitis and HIV in Africa. AIDS Rev 9: 25-39. Link: https://tinyurl.com/y2vu7y46
- Yu MW, Yeh SH, Chen PJ, Liaw YF, Lin CL, et al. (2005) Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst 97: 265-272. Link: https://tinyurl.com/y5xarafe
- Chen CJ, Yang HI, Su J, Jen CL, You SL, et al. (2006) Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 295: 65-73. Link: https://tinyurl.com/y6n6qd34
- Liu TT, Fang Y, Xiong H, Chen TY, Ni ZP, et al. (2012) A casecontrolstudy of the relationship between hepatitis B virus DNA level Zhou et al. Virology Journal 9: 16 http://www.virologyj.com/content/9/1/16 Page 4 of 5 and risk of hepatocellular carcinoma in Qidong, China. World J Gastroenterol 2008, 14:3059-3063.
- Ito K, Arai M, Imazeki F, Yonemitsu Y, Bekku D, et al. (2010) Risk of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Scand J Gastroenterol 45: 243-249. Link: https://tinyurl.com/yy6u5j9b
- Chan HL, Wong VW, Wong GL, Chim AM, Lai LH, et al. (2009) Evaluation of impact of serial hepatitis B virus DNA levels on development of hepatocellular carcinoma. J Clin Microbiol 47: 1830-1836. Link: https://tinyurl.com/y5szlfag
- Chen G, Lin W, Shen F, Iloeje UH, London WT, et al. (2006) Past HBV viral load as predictor of mortality and morbidity from HCC and chronic liver disease in a prospective study. Am J Gastroenterol 101: 1797-1803. Link: https://tinyurl.com/y3loe694
- Hepatitis B (2010) Hepatitis B viral load and risk for liver cirrhosis and hepatocellular carcinoma in The Gambia, West Africa. J Viral Hepat 17: 115–122. Link: https://tinyurl.com/y2ad3abw
- Marjolis HS, Alter MJ, Hadler SC (1991) Hepatitis B: Evolving epidemiology and implications for control. Semin Liver Dis 11: 84-92. Link: https://tinyurl.com/y4d4fu87
- Sana Irfan, Sahu NP, Singh SB (2015) Seroprevalance of Hepatitis B Patients Attending a Tertiary Care Hospital Of Jharkhand, India. Research Journal of Pharmaceutical, Biological and Chemical Sciences 6: 147-152 Link: https://tinyurl.com/y63u2fbe
- McMahon BJ, Holck P, Bulkow L, Snowball M (2001) Serologic and clinical outcomes of 1536 Alaska natives chronically infected with hepatitis B virus. Ann Intern Med 135: 759-768. Link: https://tinyurl.com/y6mmx64l
- Chen CJ, Liang KY, Chang AS, Chang YC, Lu SN, et al. (1991) Effects of hepatitis B virus, alcohol drinking, cigarette smoking and familial tendency on hepatocellular carcinoma. Hepatology 13: 398-406. Link: https://tinyurl.com/y56fqhhr
- Tsai JF, Jeng JE, Ho MS, Chang WY, Hsieh MY, et al. (1996) Additive effect modification of hepatitis B surface antigen and e antigen on the development of hepatocellular carcinoma. Br J Cancer 73: 1498-1502. Link: https://tinyurl.com/yybojphg
- Yang HI, Lu SN, Liaw YF, You SL, Sun CA, et al. (2002) Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med 347: 168-174. Link: https://tinyurl.com/yyljyb6m
- Qu LS, Liu TT, Jin F, Guo YM, Chen TY, et al. (2011) Combined pre-S deletion and core promoter mutations related to hepatocellular carcinoma: A nested case-control study in China. Hepatol Res 41: 54-63. Link: https://tinyurl.com/y3h69c8q
- Wu CF, Yu MW, Lin CL (2008) Long-term tracking of hepatitis B viral load and the relationship with risk for hepatocellular carcinoma in men. Carcinogenesis 29: 106-112. Link: https://tinyurl.com/y5xw3dp3
- Chen G, Lin W, Shen F (2006) Past HBV viral load as predictor of mortality and morbidity from HCC and chronic liver disease in a prospective study. Am J Gastroenterol 101: 1797-1803. Link: https://tinyurl.com/y3loe694
- Chien RN, Lin CH, Liaw YF (2003) The effect of lamivudine therapy in hepatic decompensation during acute exacerbation of chronic hepatitis B. J Hepatol 38: 322-327. Link: https://tinyurl.com/yyhe8rom
- Ari BZ, Zemel R, Kazetsker A, Fraser G, Kaspa TR (1999) Efficacy of lamivudine in patients with hepatitis B virus precore mutant infection before and after liver transplantation. Am J Gastroenterol 94: 663-667. Link: https://tinyurl.com/y46wgn5r
- Locarnini S (2004) Molecular Virology of Hepatitis B Virus. Semin Liver Dis 24: 3-10. Link: https://tinyurl.com/y5f39k2r
- Liao Y, Hu X, Chen J, Cai B, Tang J, et al. (2012) Precore mutation of hepatitis B virus may contribute to hepatocellular carcinoma risk: evidence from an updated meta analysis. PloS ONE. Link: https://tinyurl.com/y52v6lpn
- Hasegawa K, Huang J, Wand JR, Obata H, Liang TJ (1991) Association of hepatitis B viral precore mutations with fulminant hepatitis B in Japan. Virology 185: 460-463. Link: https://tinyurl.com/yy67znm4
- Liang TJ, Hasegawa K, Munoz SJ, Shapiro CN, Yoffe B, et al. (1994) Hepatitis B virus pre-core mutation and fulminant hepatitis in the United States: a polymerase chain reaction-based assay for the detection of specific mutation. J Clin Invest 93: 550-555. Link: https://tinyurl.com/y3txp6xz
- Liu CJ, Cheng HR, Chen CL, Chen TC, Tseng TC, et al. (2011) Effects of hepatitis B virus precore and basal core promoter mutations on the expression of viral antigens: genotype B vs C. J Viral Hepat 18: e482-490. Link: https://tinyurl.com/y2vef9rf
- Vivekanandan P, Bissett S, Ijaz S, Teo CG, Sridharan G, et al. (2008) Correlation between hepatitis B genotypes, 1896 precore mutation, virus loads and liver dysfunction in an Indian population. Indian J Gastroenterol 27: 142-147. Link: https://tinyurl.com/yya7os65
- Liu CJ, Chen BF, Chen PJ, Lai MY, Huang WL, et al. (2006) Role of hepatitis B viral load and basal core promoter mutation in hepatocellular carcinoma in hepatitis B carriers. J Infect Dis 193: 1258-1265. Link: https://tinyurl.com/y65tb5ts
- Chen CJ, Yang HI, Su J, Jen CL, You SL, et al. (2006) Risk of hepatocellular carcinoma across a biological gradient of serum. JAMA 295: 65-73. Link: https://tinyurl.com/y6n6qd34
- Apurva AM, Jordan JF (2007) Viral hepatitis and HIV in Africa. AIDS Reviews 9: 25-39. Link: https://tinyurl.com/y2vu7y46
- Asim M, Malik A, Sarma MP, Polipalli SK, Begum N, et al. (2010) Hepatitis B virus BCP, Precore/core, × gene mutations/genotypes and the risk of hepatocellular carcinoma in India. J Med Virol 82: 1115-1125. Link: https://tinyurl.com/y3sejczj
- Yu MW, Yeh SH, Chen PJ, Liaw YF, Lin CL, et al. (2005) Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst 97: 265-272. Link: https://tinyurl.com/y5xarafe
- Chen CJ, Yang HI, Su J, Jen CL, You SL, et al. (2006) Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 295: 65-73. Link: https://tinyurl.com/y6n6qd34
- Ito K, Arai M, Imazeki F, Yonemitsu Y, Bekku D, et al. (2010) Risk of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Scand J Gastroenterol 45: 243-249. Link: https://tinyurl.com/yy6u5j9b
- Chen CJ, Yang HI, Su J, Jen CL, You SL, et al. (2006) Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 295: 65-73. Link: https://tinyurl.com/y6n6qd34
- Liu TT, Fang Y, Xiong H, Chen TY, Ni ZP, et al. (2012) A casecontrolstudy of the relationship between hepatitis B virus DNA level Zhou et al. Virology Journal 2012, 9:16 http://www.virologyj.com/content/9/1/16 Page 4 of 5 and risk of hepatocellular carcinoma in Qidong, China. World J Gastroenterol 2008, 14:3059-3063.
- Ito K, Arai M, Imazeki F, Yonemitsu Y, Bekku D, et al. (2010) Risk of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Scand J Gastroenterol 45: 243-249.
- Chan HL, Wong VW, Wong GL, Chim AM, Lai LH, et al. (2009) Evaluation of impact of serial hepatitis B virus DNA levels on development of hepatocellular carcinoma. J Clin Microbiol 47: 1830-1836. Link: https://tinyurl.com/y5szlfag
- Chen G, Lin W, Shen F, Iloeje UH, London WT, et al. (2006) Past HBV viral load as predictor of mortality and morbidity from HCC and chronic liver disease in a prospective study. Am J Gastroenterol 101: 1797-1803. Link: https://tinyurl.com/y3loe694
- (2010) Hepatitis B viral load and risk for liver cirrhosis and hepatocellular carcinoma in The Gambia, West Africa. Mendy ME, Welzel T, Lesi OA, Hainaut P, Hall AJ, et al. Journal of Viral Hepatitis 17: 115–122. Link: https://tinyurl.com/y5gkj9gs
- Chen G, Lin W, Shen F (2006) Past HBV viral load as predictor of mortality and morbidity from HCC and chronic liver disease in a prospective study. Am J Gastroenterol 101: 1797–1803. Link: https://tinyurl.com/y3loe694
- Iloeje UH, Yang HI, Su J, Jen CL, You SL, et al. (2006) Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 130: 678–686. Link: https://tinyurl.com/y5wsjfjr
- Yu SJ, Kim YJ (2014) Hepatitis B viral load affects prognosis of hepatocellular Carcinoma. World J gastroenterology 14; 20: 12039-12044. Link: https://tinyurl.com/y3bqsjec
- Chan HL, Tse CH, Mo F, Koh J, Wong VW, et al. (2008) High viral load and hepatitis B virus subgenotype ce are associated with increased risk of hepatocellular carcinoma. J Clin Oncol 26: 177-182. Link: https://tinyurl.com/y5hhhn2x
- Chen CJ, Yang HI, Su J, Jen CL, You SL, et al. (2006) Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 295: 65–73. Link: https://tinyurl.com/y6n6qd34
- Iloeje UH, Yang HI, Su J, Jen CL, You SL, et al. (2006) Predicting liver cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 130: 678–686. Link: https://tinyurl.com/y5wsjfjr
- Yuen MF, Sablon E, Wong DK, Yuan HJ, Chun-Yu Wong B (2003) Role of hepatitis B virus genotypes in chronic hepatitis B exacerbation. Clin Infect Dis 37: 593-597. Link: https://tinyurl.com/yyf7pd8c
- Chan HL, Tsang SW, Hui Y (2002) The role of lamivudine and predictors of mortality in severe flare-up of chronic hepatitis B with jaundice. J Viral Hepat 9: 424-428. Link: https://tinyurl.com/yyqebswy
- Hadler SC, Judson FN, O’Malley PM, Altman NL, Penley K, et al. (1991) Outcome of hepatitis B virus infection in homosexual men and its relation to prior human immunodeficiency virus infection. J Infect Dis 163: 454-459. Link: https://tinyurl.com/y2mrabx9
- Thio CL (2003) Hepatitis B in the human immunodeficiency virus-infected patient: epidemiology, natural history, and treatment. Semin Liver Dis 23: 125-136. Link: https://tinyurl.com/y5yps7gv
- Bica I, McGovern B, Dhar R, Stone D, McGowan K, et al. (2001) Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis 32: 492-497. Link: https://tinyurl.com/y567kqhw
- Thio CL, Seaberg EC, Skolasky R (2002) HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 360: 1921-1926. Link: https://tinyurl.com/y6oxau47
- Soriano V, Puoti M, Bonacini M (2005) Care of patients with chronic hepatitis B and HIV co-infection: recommendations from an HIV-HBV International Panel. Aids 19: 221-240. Link: https://tinyurl.com/y6qb8dls
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
