Archives of Hepatitis Research
The association with histopathological findings and predictive significance of transforming growth factor beta 1 (TGF β1) in patients with chronic viral hepatitis
Mehmet Sait Bugdaci1*, Cetin Karaca2, Ali Rıza Koksal1, Salih Boga1, Canan Alkim1 and Mehmet Sokmen1
2Gastroenterohepatology, Department of Internal Medicine, Istanbul University Istanbul Medical School, Turkey
Cite this as
Bugdaci MS, Karaca C, Koksal AR, Boga S, Alkim C, et al. (2023) The association with histopathological findings and predictive significance of transforming growth factor beta 1 (TGF β1) in patients with chronic viral hepatitis. Arch Hepat Res 9(1): 005-010. DOI: 10.17352/ahr.000035Copyright License
© 2023 Bugdaci MS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: Chronic Viral Hepatitis (CVH) is the most common cause of hepatocellular cancer and cirrhosis related to liver fibrosis. The gold standard in the diagnosis of fibrosis is liver biopsy. TGF β1 is a pleiotropic cytokine that plays a pivotal role in carcinogenesis and fibrosis. The results of studies investigating the relationship between TGF β1 and histopathological findings are controversial. We aimed to investigate the relationship between TGF β1 and histopathological findings.
Methods: Patients with Chronic Hepatitis B (CHB) and C (CHC), Non-Alcoholic Steatohepatitis (NASH), inactive HBsAg carriers, patients with cirrhosis and healthy control cases presenting to the Gastroenterology Clinic of Sisli Etfal Training and Research Hospital between 2009-2010 were included in the study. Laboratory tests, HCV RNA, HBV DNA, viral load, and viral markers (such as HBsAg, anti-HCV) were determined. Biopsies were performed on patients with hepatitis B and C, and non-alcoholic steatohepatitis (NASH). Histologic features were defined as Histologic Activity Index (HAI) and fibrosis stage (Knodell’s scoring). TGF β1 was evaluated by the ELISA method.
Results: 267 cases including 44 non-alcoholic steatohepatitis cases [27 female (57%)], 38 inactive HBsAg carriers [23 female (60%)], 48 patients with chronic hepatitis B [17 female (35%)], 27 chronic hepatitis C [14 female (60%)], 15 decompensated cirrhosis [3 female (20%)] and 94 healthy control cases were included in the study. Compared with healthy controls, all other subgroups had significantly elevated TGF β1 levels. TGF β1 was found to have a specificity of 93.6% and a sensitivity of 88.9% (AUC: 0.948, 95% CI: 0.916-0.981) in determining liver diseases. TGF β1 had a positive correlation with fibrosis and histological activity index in patients with CHB and CHC. There was a negative correlation between TGF β1 and HBV DNA and HCV RNA. TGF β1 had a significant correlation with LDL and total cholesterol in cases with CHB and CHC.
Conclusion: TGF β1 is correlated with both HAI and fibrosis in patients with CHB and CHC. TGF β1 might have a role in the prognostic significance of elevated LDL levels and low viral load in patients with CHC.
Introduction
Chronic Viral Hepatitis (CVH) due to hepatitis B and C is the leading cause of chronic hepatitis, cirrhosis, and hepatocellular cancer. Therefore, CVH has increased morbidity and mortality rates [1,2].
The basic histopathological finding in the liver of patients with CVH is necroinflammation with its pathologic definition of histologic activity index (HAI) and fibrosis. Necroinflammation is the major criterion for the initiation of therapy in CVH cases [3]. Moreover, hepatic fibrosis, caused by necroinflammation and leading to cirrhosis, has been demonstrated to be reversible [4,5]. An improvement in the fibrosis stage has been reported to prevent the development of cirrhosis and hepatocarcinogenesis [6]. Therefore, pre-and post-treatment assessment of the fibrosis and the other histopathological findings are important with respect to the efficacy of the treatment and the disease progression. However, the current gold standard diagnostic method is liver biopsy, which carries a high morbidity and mortality risk and also has limitations such as inter-observer variability, sampling errors, and contraindication in patients with bleeding diathesis [7,8].
Tests used in the diagnosis and follow-up of CVH such as transaminase levels, viral markers (e.g., HBsAg, anti-HCV), and viral load are not correlated with liver necroinflammation and fibrosis [9-11]. Therefore, it is clear that diagnostic and follow-up tests correlated to the hepatic histopathological findings are required in patients with CVH.
TGF β1 is the major component with significant functions among the TGF β isomers in the human liver [12]. TGF β1 is a pleiotropic, multifunctional marker that is pivotal in hepatic fibrogenesis through the production of excessive extracellular matrix by the activation of hepatic stellate cells and carcinogenesis [7,13]. It has also been reported to have a critical role in the termination of hepatocellular proliferation and apoptosis [14]. Therefore, TGF β1 may be related to liver fibrosis and necroinflammation.
The results from the trials investigating the correlation of TGF β1 with the histopathological findings are controversial. [15,16]. In this study, we aimed to investigate the importance of TGF β1 in liver disease, and its relationship with histopathology and laboratory parameters (lipid panel and viral load) in patients with CVH.
Methods
Patients
Patients with chronic hepatitis B and C presenting to the Gastroenterology Clinic of Sisli Etfal Training and Research Hospital in 2009-2010 were included in the study. Chronic Hepatitis C (CHC) infection was defined as HCV RNA positivity for more than 6 months. All patients with hepatitis B included in the study were HBeAg negative. Chronic Hepatitis B (CHB) was defined as persistent or intermittent transaminase elevation and serum HBV DNA levels over 2000 IU/mL in patients with HBsAg positivity for more than 6 months. Inactive HBsAg carriers were defined as persistently normal transaminase levels and serum HBV DNA levels below 2000 IU/mL in patients with HBsAg positivity for more than 6 months. Nonalcoholic Fatty Liver Disease (NAFLD) was defined as hepatosteatosis by biopsy and/or sonography with transaminase levels above the normal range. A diagnosis of cirrhosis was established using histologic criteria or by clinical and Doppler sonographic findings.
Exclusion criteria
Patients with acute inflammation (elevated CRP and sedimentation), use of angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists, coronary artery disease, essential and secondary thrombocytosis, history of thrombasthenia, hepatocellular carcinoma, portal vein thrombosis, alcoholism, viral co-infections (such as hepatitis D, human immunodeficiency virus, B plus C hepatitis) and systemic diseases (chronic renal failure, nephrotic and nephritic syndrome), and acute renal failure were excluded from the study.
HBV DNA and HCV RNA viral load assay and evaluation of other tests
Sera for the complement assay were collected from patients before commencing treatment. HBV DNA quantitation was made with the COBAS TaqMan HBV Monitor test (Roche Molecular Systems) in accordance with the manufacturer’s instructions. The dynamic interval for HBV DNA detection was 6- 110.000.000 IU/ml. HBsAg, AntiHIV, AntiHCV, and Anti delta were assayed using ETI-MAK-4, ETI-AB-HCVK-4, and ETI-AB-DELTAK-2 kits respectively with a TİMAX (DiaSorin, Italy) device. AntiHBc IgG, AntiHBc IgM, and HBeAg tests were carried out with a DiaSorin LIAISON system. As all the genotype studies in Turkey show a predominance of genotype D HBV, we did not perform a genotype analysis [17-19]. Patients gave blood samples for HCV RNA testing to determine viral load before the initiation of treatment. HCV-RNA quantitation was done using COBAS Taqman HCV test v2.0 (Roche Molecular Systems) in accordance with the manufacturer’s instructions. The dynamic range for HCV-RNA quantitation was 6-110,000,000 copies/ml. All patients with chronic hepatitis C were genotype 1b. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), albumin, C-reactive protein (CRP), erythrocyte sedimentation rate, complete blood count, urine analysis and urinary protein quantification were performed for all patients. ALT cut-offs were 30 IU/ L and 19 IU/L in males and females, respectively [20].
Evaluation of TGF β1 by Enzyme-Linked Immunosorbent Assay (ELISA)
Human TGF-β1 Enzyme-Linked Immunosorbent Assay (ELISA) (Bender MedSystems, GmbH, Vienna, Austria, Europe) was used for the detection and quantification of TGF-β1 according to the manufacturer’s instructions. Briefly, serum samples were diluted 1:10 and incubated with 1N HCL for 1 hour at room temperature, and then neutralized with 1N NaOH. 1:2 serial dilutions of human TGF-β1 standard ranging between 30.00 and 0.47 ng/ml were prepared for the standard curve. ELISA was performed in 96-well plates that were pre-coated with a polyclonal antibody specific for human TGF-β1. Pretreated serum samples and serial dilutions of human TGF-β1 standard were added to the microplate; then the conjugate was added to each well and the plate was incubated for 4 hours at room temperature. The rest of the reaction steps were carried out on an automated ELISA analyzer (ETIMAX, Diasorin) which includes washing, pipetting substrate, incubation, stopping the reaction, and reading the absorbance of each microwell on spectro-photometer using 450 nm as the primary wavelength and 620 nm as a reference. The cytokine protein concentration of each sample was determined on a standard curve generated by performing parallel assays using known amounts of TGF-β1 standards by using absorbance values of samples. TGF β1 content in the liver tissue was not evaluated as previous studies have demonstrated a strong correlation between hepatic and plasma TGF β1 [21].
Evaluation of liver histology
A liver biopsy was performed using a liver biopsy needle after obtaining informed consent from the patients (length of specimen > 2 cm). Biopsy samples were fixed in buffered formaldehyde for 24 hours and then processed by routine procedures. Liver biopsy samples were evaluated by a pathologist blind to clinical and virological findings with hematoxylin and eosin stained sections and periodic acid-Schiff stain with diastase for necroinflammatory activity. Masson’s trichrome and Sweet’s reticulin stains were reviewed for fibrosis and structural change. Histologic features were defined as histologic activity index (HAI, grade) and fibrosis (stage) according to the scoring method described by Knodell, et al. [22].
Ethics
The study protocol was approved by the local ethics committee and written consent was obtained from all patients prior to inclusion in the study.
Statistics
Scale variables were presented as mean ± standard deviation (mean ± SD). Categorical data were evaluated using Chi-square analysis or with Spearman’s correlation as appropriate. Parametric quantitative data were compared by Student’s t-test or Pearson’s correlation as appropriate. One-way analysis of variance (ANOVA) was used for comparisons between the 5 main groups. Post-hoc analyses of significant differences were performed using Tamhane’s test. A group of healthy controls was tested to determine a diagnostic cut-off in liver diseases for TGF β1 using Receiver Operating Characteristics (ROC) analysis. The Mann-Whitney U test was used for inter-group comparisons (a corrected p-value of 0.008 was considered significant because multiple comparisons were performed). A p - value of < 0.05 was considered statistically significant. SPSS (Statistical Package for Social Sciences, for Windows, release 12.0.0 standard version) software was used for statistical evaluations. Medcalc 11.3.0.0 for Windows was used for the depiction of TGF β1 changes on a box-and-whiskers plot.
Results
A total of 267 patients including 44 nonalcoholic steatohepatitis [27 female (57%)] of mean age 37 ± 11 years, 38 inactive HBsAg carriers [23 female (60%)] of mean age 42 ± 12 years, 48 chronic hepatitis B patients [17 female (35%)] of mean age 43.5 ± 47 years, 27 chronic hepatitis C patients [14 female (60%)] of mean age 56 ± 6.7 years, 15 decompensated cirrhosis patients [3 female (20%)] of mean age 62 ± 9 years and 94 healthy control cases were included in the study.
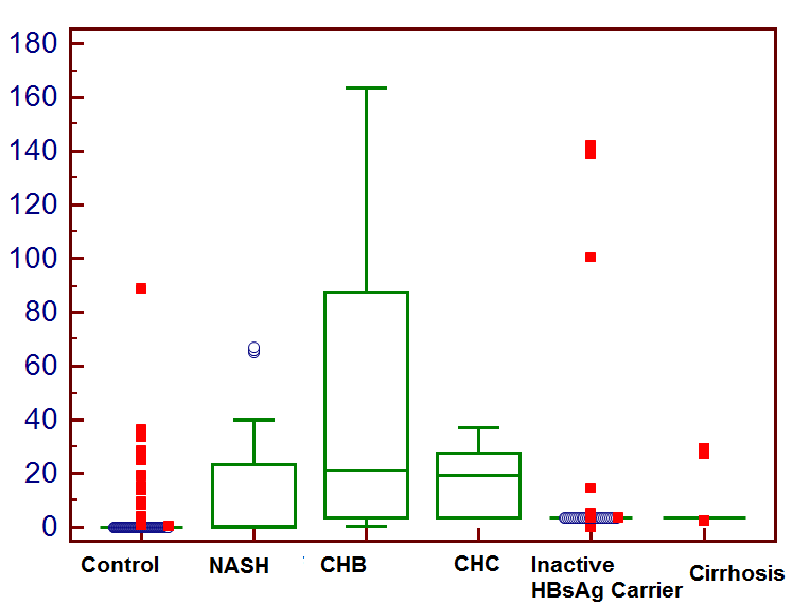
Serum TGF β1 levels were 15.5 ± 21 ng/ml in NAFLD cases, 13.3 ± 34.25 ng/ml in inactive HBsAg carriers, 43.5 ± 47 ng/ml in CHB cases, 17.88 ± 13.3 ng/ml in CHC cases, 6.6 ± 8.74 ng/ml in patients with cirrhosis and 0.82 ± 2.83 ng/ml in healthy controls (Figure 1).
When compared with the control group, serum TGF β1 levels were significantly higher in patient groups with NAFLD (p < 0.001), inactive HBsAg carriers (p < 0.001), CHB (p < 0.001), CHC (p < 0.001) and cirrhosis (p < 0.001). Serum TGF β1 levels were significantly elevated in CHB cases, NAFLD (p = 0.004), inactive HBsAg carriers (p < 0.001), and cirrhosis cases (p = 0.001), but there was no difference among CHC cases (p = 0.219).
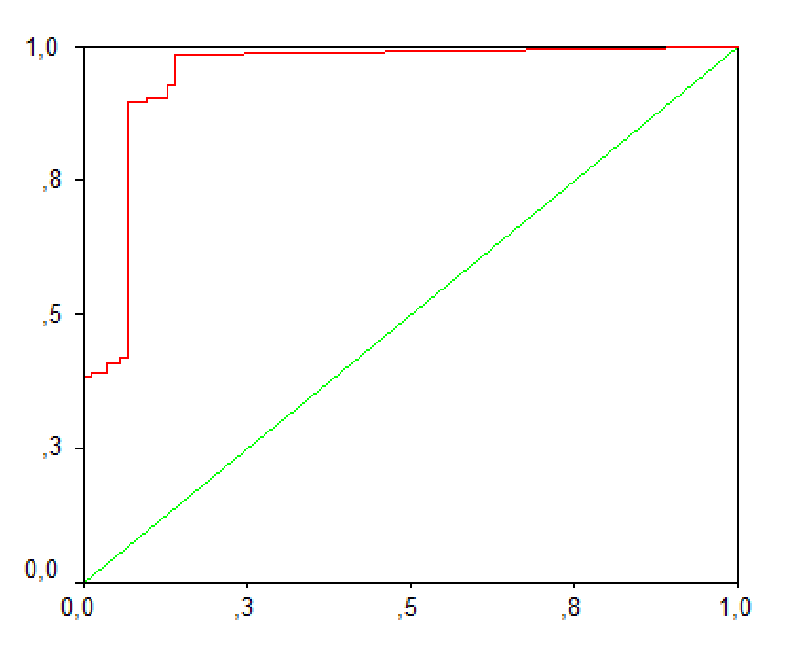
When compared with the control group, in cases where TGF β1 levels were above the upper normal limit of 3.46 ng/ml, the specificity of TGF β1 for the determination of liver disease was 93.6%, and its sensitivity was 88.9% (AUC: 0.948, 95% CI: 0.916-0.981) (Figure 2).
Upon comparison of mean lipid levels in patients with CHB and CHC, there was no statistically significant difference in terms of total cholesterol (P = 0.483), HDL (p = 0.133), LDL (p = 0.678), and triglyceride (p = 0.567) levels.
Demographic and laboratory data pertaining to patients with CHB and CHC included in the study are summarized in Tables 1,2.
Discussion
High serum TGF β1 levels in patients with chronic viral hepatitis have been reported by the vast majority of authors [23-25]. In this study, we compared liver-related pathologies with high TGF β1 levels (NAFLD, CHC, CHB, cirrhosis, inactive HBsAg carriers) with the control group. According to this, we found serum TGF β1 levels higher than the upper normal range of 3.46 ng/ml established by the manufacturer to have a specificity of 93.6% and sensitivity of 88.9% in determining liver disease. Hence, the assessment of the TGF β1 level as well as the measurement of the transaminases, which clinically represent nonspecific hepatic tests, may provide further benefit.
In the present study, we demonstrated a correlation between TGF β1 and the stage of fibrosis in patients with CHC and CHB. Several authors have reported a correlation between TGF β1 and fibrosis [16,23,26]. While the exact mechanism underlying the increase in TGF β1 in cases with CVH is not known, the HCV core protein was reported to potentially promote hepatic fibrogenesis by the upregulation of the TGF β1 [27]. Although mean serum TGF β1 levels were lower in patients with HCV, there was no statistically significant difference compared to patients with CHB. The lower levels of TGF β1 in patients with CHC may be due to the lower average stage of fibrosis in these patients. This result also corroborates the significant correlation between TGF β1 and fibrosis.
Our finding of a negative correlation with viral load in addition to a significant correlation with inflammatory grade suggests that besides the antifibrotic effect, there might also be anti-inflammatory and antiviral effects, especially in patients with CHC [28-30]. It has been reported that a positive correlation between TGF β1 and inflammatory grade [31,32] as well as opposite to this correlation [26]. The high TGF β1 levels in cases with acute viral hepatitis demonstrated by Flisiak, et al supports its association with the active hepatic inflammation and thus the grade of inflammation because the advanced stage of fibrosis is not an expected manifestation in acute viral hepatitis [33]. The detection of a correlation between TGF β1 and the scoring of the active inflammation grade is an expected finding, considering the fact that it stimulates the chemotaxis of the macrophages and the granulocytes and the release of the cytokines such as IL-1 and IL-6 [13].
In the current study, we found a significant negative correlation between TGF β1 and viral load (HBV DNA and HCV RNA). The exact reason for the negative correlation with the viral load is not known. This negative correlation may be associated with the fact that TGF β1 suppresses the proteins expressed by the HCV replicon and the viral RNA replication [34], stimulates apoptosis [35] and increases the vial clearance [36] in cases with CHC. This apoptotic effect of TGF β1, a pleiotropic cytokine, may be more pronounced in cells infected by the virus.
We found a significant positive correlation between TGF β1 and LDL and total cholesterol levels. LDL elevation has been reported as a good prognostic indicator in patients with hepatitis C [37]. However, currently, the reason for this is not clearly established. Interferon alpha-2b was reported to increase the TGF β1 level [38,39] and the cases achieving a sustained virological response (SVR) were reported to experience an increase in LDL and total cholesterol levels during treatment [37]. Thus, TGF β1 may have a role in the increase of LDL and total cholesterol levels. The results from the current study suggest that this increase in the LDL and the total cholesterol levels observed in patients achieving an SVR response may be associated with the increased synthesis of TGF β1 induced by interferon.
The limitations of this study include the absence of an assessment of TGF β1’s correlation with the therapeutical response during the course of chronic hepatitis, the absence of a determination of the TGF β1 receptors (particularly type II receptors) and the lack of an assessment of the correlation between the level of TGF β1 in the liver tissue and the serum TGF β1 level. However, since a highly significant correlation was demonstrated between the level of TGF β1 in the liver tissue and the serum TGF β1 level, such an association was not separately evaluated [21,40].
In conclusion, TGF β1 is highly sensitive and specific in determining liver diseases. In cases of CVH, the assessment of TGF β1 may provide additional benefits due to its significant correlation with the histopathological stage and grade, as opposed to the diagnostic and follow-up markers. TGF β1 might play an essential role in the increased serum LDL and low viral load previously reported as good prognostic factors in patients with CHC.
We would like to express our gratitude to Roche Pharmaceuticals for their contribution to the supply of the TGF β1 kit. Roche Pharmaceuticals had no internal or external interference that would affect the course and the results of this trial. As can be seen in the original text, the study results provided no secondary gain for Roche Pharmaceuticals.
- Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008 Oct 2;359(14):1486-500. doi: 10.1056/NEJMra0801644. Erratum in: N Engl J Med. 2010 Jul 15;363(3):298. PMID: 18832247.
- Lauer GM, Walker B. Hepatıtıs C vırus ınfectıon N Engl J Med. July 5, 2001; 345:1.
- Paradis V, Mathurin P, Laurent A, Charlotte F, Vidaud M, Poynard T, Hoang C, Opolon P, Bedossa P. Histological features predictive of liver fibrosis in chronic hepatitis C infection. J Clin Pathol. 1996 Dec;49(12):998-1004. doi: 10.1136/jcp.49.12.998. PMID: 9038738; PMCID: PMC499649.
- Tangkijvanich P, Yee HF Jr. Cirrhosis--can we reverse hepatic fibrosis? Eur J Surg Suppl. 2002;(587):100-12. PMID: 16144208.
- Yoshiji H, Noguchi R, Kojima H, Ikenaka Y, Kitade M, Kaji K, Uemura M, Yamao J, Fujimoto M, Yamazaki M, Toyohara M, Mitoro A, Fukui H. Interferon augments the anti-fibrotic activity of an angiotensin-converting enzyme inhibitor in patients with refractory chronic hepatitis C. World J Gastroenterol. 2006 Nov 14;12(42):6786-91. doi: 10.3748/wjg.v12.i42.6786. PMID: 17106926; PMCID: PMC4087432.
- Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology. 2002 May;122(6):1609-19. doi: 10.1053/gast.2002.33411. PMID: 12016426.
- Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38-53. doi: 10.1016/s0168-8278(02)00429-4. PMID: 12591185.
- Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002 Oct;97(10):2614-8. doi: 10.1111/j.1572-0241.2002.06038.x. PMID: 12385448.
- Shao J, Wei L, Wang H, Sun Y, Zhang LF, Li J, Dong JQ. Relationship between hepatitis B virus DNA levels and liver histology in patients with chronic hepatitis B. World J Gastroenterol. 2007 Apr 14;13(14):2104-7. doi: 10.3748/wjg.v13.i14.2104. PMID: 17465456; PMCID: PMC4319133.
- Choi Y, Putti T, Win K, Hu Y, Remy P, Bloom A. Correlation of viral RNA, alanine aminotransferase, and histopathology in hepatitis C virus-associated hepatitis. Mol Diagn. 1999 Sep;4(3):251-4. doi: 10.1016/s1084-8592(99)80029-0. PMID: 10553026.
- Lee YS, Yoon SK, Chung ES, Bae SH, Choi JY, Han JY, Chung KW, Sun HS, Kim BS, Kim BK. The relationship of histologic activity to serum ALT, HCV genotype and HCV RNA titers in chronic hepatitis C. J Korean Med Sci. 2001 Oct;16(5):585-91. doi: 10.3346/jkms.2001.16.5.585. PMID: 11641527; PMCID: PMC3057604.
- Liu YG, Lu JH, Wang XX, Yang JL, Lang ZW, Meng X, Zhang LJ, Sun L, Zhang SJ, Li JQ, Song CZ. Influence of HBcAg in liver cell plasma on expression of transforming growth factor-beta 1 in liver tissue of low-grade chronic hepatitis B patients. World J Gastroenterol. 2006 Jan 7;12(1):127-9. doi: 10.3748/wjg.v12.i1.127. PMID: 16440431; PMCID: PMC4077501.
- Marek A, Brodzicki J, Liberek A, Korzon M. TGF-beta (transforming growth factor-beta) in chronic inflammatory conditions - a new diagnostic and prognostic marker? Med Sci Monit. 2002 Jul;8(7):RA145-51. PMID: 12118214.
- Tsuchiya S, Tsukamoto Y, Taira E, LaMarre J. Involvement of transforming growth factor-beta in the expression of gicerin, a cell adhesion molecule, in the regeneration of hepatocytes. Int J Mol Med. 2007 Mar;19(3):381-6. PMID: 17273784.
- Lebensztejn DM, Sobaniec-Lotowska M, Kaczmarski M, Werpachowska I, Sienkiewicz J. Serum concentration of transforming growth factor (TGF)-beta 1 does not predict advanced liver fibrosis in children with chronic hepatitis B. Hepatogastroenterology. 2004 Jan-Feb;51(55):229-33. PMID: 15011870.
- El Bassuoni MA, Talaat RM, Ibrahim AA, Shaker OT. TGF-beta1 and C-erb-B2 neu oncoprotein in Egyptian HCV related chronic liver disease and hepatocellular carcinoma patients. Egypt J Immunol. 2008;15(1):39-50. PMID: 20306668.
- Ozdemir FT, Duman D, Ertem D, Avşar E, Eren F, Ozdoğan O, Kalayci C, Aslan N, Bozdayi AM, Tözün N. Determination of hepatitis B genotypes in patients with chronic hepatitis B virus infection in Turkey. Turk J Gastroenterol. 2005 Dec;16(4):183-7. PMID: 16547844.
- Bozdayi AM, Bozkaya H, Türkyilmaz AR, Sarýodlu M, Cetinkaya H, Karayalçin S, Yurdaydin C, Uzunalimoğlu O. Nucleotide divergences in the core promoter and precore region of genotype D hepatitis B virus in patients with persistently elevated or normal ALT levels. J Clin Virol. 2001 Apr;21(1):91-101. doi: 10.1016/s1386-6532(01)00148-2. PMID: 11255102.
- Yalcin K, Degertekin H, Bahcecioglu IH, Demir A, Aladag M, Yildirim B, Horasanli S, Ciftci S, Badur S. Hepatitis B virus genotype D prevails in patients with persistently elevated or normal ALT levels in Turkey. Infection. 2004 Feb;32(1):24-9. doi: 10.1007/s15010-004-3010-7. PMID: 15007739.
- Assy N, Beniashvili Z, Djibre A, Nasser G, Grosovski M, Nseir W. Lower baseline ALT cut-off values and HBV DNA levels better differentiate HBeAg- chronic hepatitis B patients from inactive chronic carriers. World J Gastroenterol. 2009 Jun 28;15(24):3025-31. doi: 10.3748/wjg.15.3025. PMID: 19554656; PMCID: PMC2702111.
- Tsushima H, Kawata S, Tamura S, Ito N, Shirai Y, Kiso S, Doi Y, Yamada A, Oshikawa O, Matsuzawa Y. Reduced plasma transforming growth factor-beta1 levels in patients with chronic hepatitis C after interferon-alpha therapy: association with regression of hepatic fibrosis. J Hepatol. 1999 Jan;30(1):1-7. doi: 10.1016/s0168-8278(99)80001-4. PMID: 9927144.
- Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol. 2007 Oct;47(4):598-607. doi: 10.1016/j.jhep.2007.07.006. Epub 2007 Jul 30. PMID: 17692984.
- Nelson DR, Gonzalez-Peralta RP, Qian K, Xu Y, Marousis CG, Davis GL, Lau JY. Transforming growth factor-beta 1 in chronic hepatitis C. J Viral Hepat. 1997 Jan;4(1):29-35. doi: 10.1046/j.1365-2893.1997.00124.x. PMID: 9031062.
- Neuman MG, Benhamou JP, Malkiewicz IM, Ibrahim A, Valla DC, Martinot-Peignoux M, Asselah T, Bourliere M, Katz GG, Shear NH, Marcellin P. Kinetics of serum cytokines reflect changes in the severity of chronic hepatitis C presenting minimal fibrosis. J Viral Hepat. 2002 Mar;9(2):134-40. doi: 10.1046/j.1365-2893.2002.00343.x. PMID: 11876796.
- Yasmin Anum MY, Looi ML, Nor Aini AH, Merican I, Wahidah A, Mohd Radzi AH, Nor Azizah A, Othman NH. Combined assessment of TGF-beta-1 and alpha-fetoprotein values improves specificity in the diagnosis of hepatocellular carcinoma and other chronic liver diseases in Malaysia. Med J Malaysia. 2009 Sep;64(3):223-7. PMID: 20527273.
- Flisiak R, Al-Kadasi H, Jaroszewicz J, Prokopowicz D, Flisiak I. Effect of lamivudine treatment on plasma levels of transforming growth factor beta1, tissue inhibitor of metalloproteinases-1 and metalloproteinase-1 in patients with chronic hepatitis B. World J Gastroenterol. 2004 Sep 15;10(18):2661-5. doi: 10.3748/wjg.v10.i18.2661. PMID: 15309715; PMCID: PMC4572189.
- Shin JY, Hur W, Wang JS, Jang JW, Kim CW, Bae SH, Jang SK, Yang SH, Sung YC, Kwon OJ, Yoon SK. HCV core protein promotes liver fibrogenesis via up-regulation of CTGF with TGF-beta1. Exp Mol Med. 2005 Apr 30;37(2):138-45. doi: 10.1038/emm.2005.19. PMID: 15886528.
- Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008 May 8;453(7192):236-40. doi: 10.1038/nature06878. Epub 2008 Mar 26. PMID: 18368049; PMCID: PMC2597437.
- Grainger DJ, Byrne CD, Witchell CM, Metcalfe JC. Transforming growth factor beta is sequestered into an inactive pool by lipoproteins. J Lipid Res. 1997 Nov;38(11):2344-52. PMID: 9392432.
- Teicher BA. Transforming growth factor-beta and the immune response to malignant disease. Clin Cancer Res. 2007 Nov 1;13(21):6247-51. doi: 10.1158/1078-0432.CCR-07-1654. PMID: 17975134.
- Bedossa P, Poynard T, Mathurin P, Lemaigre G, Chaput JC. Transforming growth factor beta 1: in situ expression in the liver of patients with chronic hepatitis C treated with alpha interferon. Gut. 1993;34(2 Suppl):S146-7. doi: 10.1136/gut.34.2_suppl.s146. PMID: 8314486; PMCID: PMC1374046.
- Piekarska A, Piekarski J, Omulecka A, Szymczak W, Kubiak R. Expression of Ki-67, transforming growth factor beta1, and B-cell lymphoma-leukemia-2 in liver tissue of patients with chronic liver diseases. J Gastroenterol Hepatol. 2006 Apr;21(4):700-10. doi: 10.1111/j.1440-1746.2006.04149.x. Erratum in: J Gastroenterol Hepatol. 2007 Sep;22(9):1556. Omulecka, Aleksandra [added]. PMID: 16677156.
- Flisiak R, Prokopowicz D, Jaroszewicz J, Flisiak I. Plasma transforming growth factor-beta(1) in acute viral hepatitis. Med Sci Monit. 2005 Jun;11(6):CR304-308. Epub 2005 May 25. PMID: 15917723.
- Murata T, Ohshima T, Yamaji M, Hosaka M, Miyanari Y, Hijikata M, Shimotohno K. Suppression of hepatitis C virus replicon by TGF-beta. Virology. 2005 Jan 20;331(2):407-17. doi: 10.1016/j.virol.2004.10.036. PMID: 15629783.
- Cheng PL, Chang MH, Chao CH, Lee YH. Hepatitis C viral proteins interact with Smad3 and differentially regulate TGF-beta/Smad3-mediated transcriptional activation. Oncogene. 2004 Oct 14;23(47):7821-38. doi: 10.1038/sj.onc.1208066. PMID: 15334054.
- Kimura T, Saito T, Yoshimura M, Yixuan S, Baba M, Ji G, Muramatsu M, Kawata S. Association of transforming growth factor-beta 1 functional polymorphisms with natural clearance of hepatitis C virus. J Infect Dis. 2006 May 15;193(10):1371-4. doi: 10.1086/503436. Epub 2006 Apr 5. PMID: 16619184.
- Corey KE, Kane E, Munroe C, Barlow LL, Zheng H, Chung RT. Hepatitis C virus infection and its clearance alter circulating lipids: implications for long-term follow-up. Hepatology. 2009 Oct;50(4):1030-7. doi: 10.1002/hep.23219. PMID: 19787818; PMCID: PMC4330996.
- Alvarez Mde L, Quiroga AD, Parody JP, Ronco MT, Francés DE, Carnovale CE, Carrillo MC. Cross-talk between IFN-alpha and TGF-beta1 signaling pathways in preneoplastic rat liver. Growth Factors. 2009 Feb;27(1):1-11. doi: 10.1080/08977190802547357. PMID: 19003557.
- Caríllo MC, Alvarez Mde L, Quiroga AD. Interferon alfa-2b triggers transforming growth factor-beta-induced apoptosis on preneoplasticliver. Ann Hepatol. 2006 Oct-Dec;5(4):244-50. PMID: 17153763.
- Marek B, Kajdaniuk D, Mazurek U, Janczewska-Kazek E, Kos-Kudla B, Strzalka B, Fila A, Niedziolka D, Beniowski M, Ostrowska Z, Borgiel-Marek H, Kajdaniuk J, Sieminska L, Nowak M, Wilczok T, Pakula D, Filipczyk P. TGF-beta1 mRNA expression in liver biopsy specimens and TGF-beta1 serum levels in patients with chronic hepatitis C before and after antiviral therapy. J Clin Pharm Ther. 2005 Jun;30(3):271-7. doi: 10.1111/j.1365-2710.2005.00644.x. PMID: 15896245.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
