Archives of Pulmonology and Respiratory Care
Sleep Apnea in COPD, the Role of Oxygen Saturation Index (ODI 4%) and the Ratio of Diaphragmatic Ultrasound
Doctor, Department of Medicine, Pulmonology Service, Constantine Regional Military Hospital, University Constantine 3, Constantine, Algeria
Author and article information
Cite this as
Meridj A, Belala R, Tlili K, Djeghri Y. Sleep Apnea in COPD, the Role of Oxygen Saturation Index (ODI 4%) and the Ratio of Diaphragmatic Ultrasound. Arch Pulmonol Respir Care. 2024; 10(1): 032-039. Available from: 10.17352/aprc.000090
Copyright License
© 2024 Meridj A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.When COPD and obstructive sleep apnea-hypopnea syndrome coexist in one individual, it’s known as overlap syndrome. In individuals with COPD, diaphragmatic function may help anticipate nocturnal oxygen desaturation. Ultrasound has been used extensively to assess the diaphragm. We aimed to investigate the effects of ultrasound-measured diaphragm excursion and contractile capacity on percutaneous oxygen saturation in COPD.
Methods: We conducted a prospective, observational study from 2021 to 2024. A total of Sixty-one consecutive patients with spirometry-confirmed stable COPD were included after obtaining informed written consent. Demographic and clinical data, spirometric values, BMI, average night-time saturation, the Oxygen Desaturation Index (ODI) of 4% and Measurement of Diaphragm Thickness (TFdi) and Excursion (EXdi) were collected for analysis.
This is a prospective study conducted at Constantine Regional Military University Hospital.
Results: The average night-time saturation was 94,63 ± 2,16% (12 patients could not perform nocturnal oximetry), The average Oxygen Desaturation Index (ODI) was 4% 2,01 ± 2,65.
There was a significant positive correlation between diaphragmatic mobility (EXdi) and the average night-time saturation (r = 0,395, p = 0,005). However
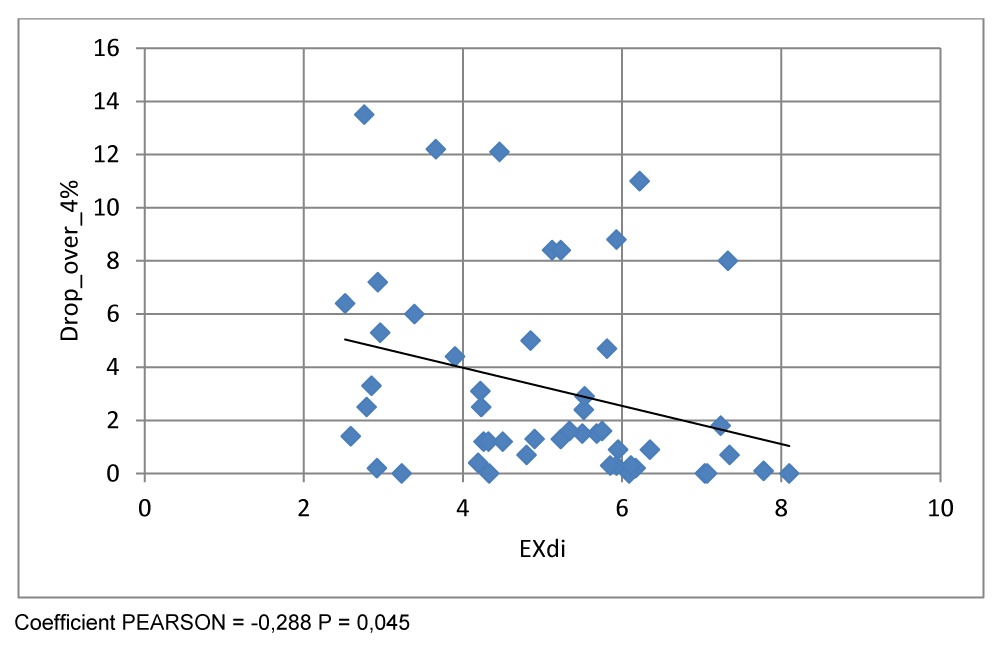
There was a significant negative correlation between diaphragmatic mobility and the average oxygen desaturation index (ODI 4 %) (r = -0,288, p = 0,045),
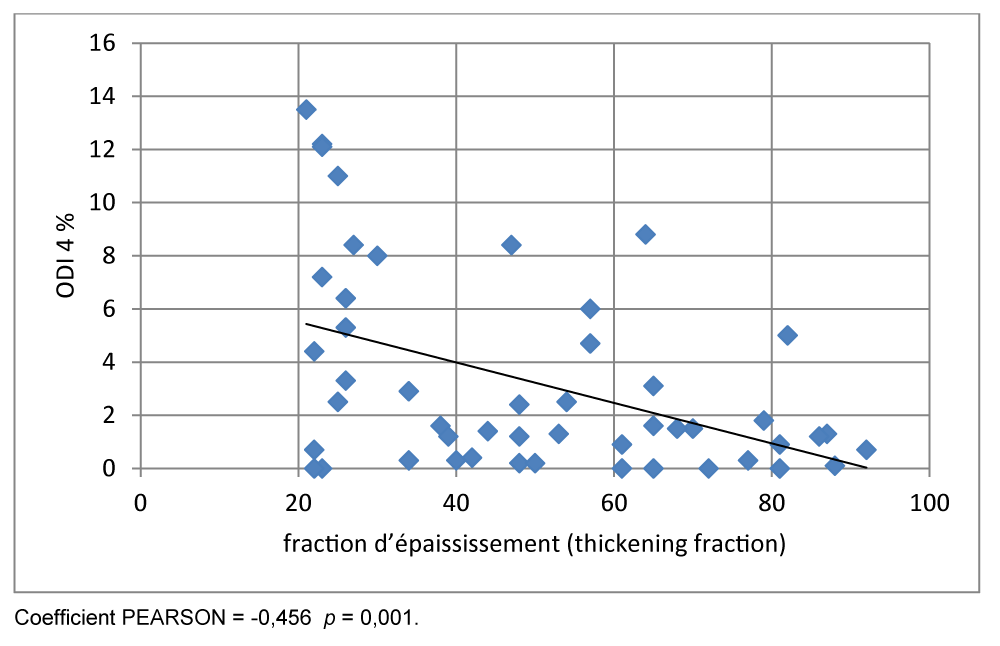
In our study on ‘objectified a significant negative correlation between 4% ODI and TFdi (r = - 0, 456 p = 0.001).
Conclusion: This study suggested a close relationship between the ultrasound parameters (TFdi, EXdi) assessed by ultrasonography in COPD on the one hand and the mean of night desaturation and (ODI) 4% on the other.
GOLD 2023 defines COPD as a heterogeneous lung condition characterized by chronic respiratory symptoms (dyspnea, cough, expectoration, and/or exacerbations) due to airway abnormalities (bronchitis, bronchiolitis) and/or alveoli (emphysema) that cause persistent, often progressive, airflow obstruction [1].
COPD is a major public health problem and is a leading cause of chronic morbidity and mortality worldwide.
The prevalence of COPD is difficult to assess because it requires patient cohorts representative of the entire population using spirometric measurements [2].
Studies have shown a wide geographic disparity in COPD prevalence [3], this is due to differences in survey methods, diagnostic criteria, and target populations.
An estimated 384 million people had COPD in 2010 and the global prevalence is estimated at 11.7% (8.4% - 15.0%) in 2015 [4].
Chronic Obstructive Pulmonary Disease (COPD) is a major public health problem and a leading cause of morbidity and mortality worldwide, according to the BOLD study [5]: 10.1% of people over 40 years old have COPD with an estimated mortality of three million deaths per year.
In Algeria, according to the BREATHE study, the prevalence of COPD is estimated at 4% in the general population and 25% among smokers [6].
Generally, the diagnosis of sleep apnea is based on polygraphy and polysomnography, ultrasound and is used for evaluation of the impact of COPD on the diaphragm and skeletal muscles of COPD, the novelty in our study is the use of ultrasound and oxygen saturation index (ODI 4%) for screening sleep apnea in patients with COPD.
Sleep apnea is a severe sleep disorder where breathing is repeatedly interrupted for long periods, enough to disrupt sleep and decrease oxygen and increase the level of carbon dioxide in the blood, 40% of COPD people report sleep disorders More recently [7], a study of 1,800 COPD patients confirmed that the majority of them complain about sleep disorders affecting their quality of life, with 66% of patients reporting night symptoms within the past month [8].
Polysomnography should be performed if sleep obstructive apnea-hypopnea syndrome (OSA) is suspected. Several authors recommend polysomnography in COPD patients with signs suggestive of OSA such as daytime sleepiness, severe pulmonary hypertension, or nycturia that is not correlated with the degree of bronchial obstruction and resting desaturation [9].
ODI 4% measures the number of times per hour when a patient’s blood oxygen level drops by 4%). It is used to suspect Sleep apnea [10,11].
During COPD, nocturnal oximetry is a less expensive and reliable tool for screening patients with severe Obstructive Sleep Apnea (OSA) due to its good agreement with the Apnea-Hypopnea Index (HIA) [12].
A study conducted by Chung, et al. [13] demonstrated that Oxygen Desaturation Index (IDO) values greater than 5, 15, and 30 were good predictors of IAH values greater than 5, 15, and 30 respectively.
Ultrasound has distinct advantages over other techniques used to assess diaphragmatic and quadricipital function, as well as COPD. For example, it is non-invasive and does not use ionizing radiation, but it is feasible, reproducible, and affordable in the patient’s bed [14].
A close relationship is expected between saturation nocturnal oxygen ODI 4% and the function of the diaphragm in COPD, but very few studies have reported of this relationship.
Heijdra, et al. [15] observed significant correlations in COPD between inspiratory muscle strength (maximal inspiratory mouth pressure and maximal transdiaphragmatic pressure) and The average night-time saturation. This research showed that inspiratory muscle strength was useful in predicting the average night-time saturation. And desaturation, but measurement of maximal transdiaphragmatic pressure, a specific indicator of diaphragmatic function, was invasive. However, ultrasound imaging, a non-invasive method, has been used widely for imaging of the diaphragm.
Objective of the study
This study suggested a close relationship between the ultrasound parameters (TFdi, EXdi) assessed by ultrasonography in COPD on the one hand and the mean of night desaturation and (ODI) 4% on the other.
Methods
Study design and participants
This is a prospective descriptive observational study conducted in the HMRUC’s Department of Pneumology, 61 patients participated in the study. According to the Global Initiative criteria for chronic obstructive pulmonary disease (GOLD).
Inclusion criteria: All patients with COPD, over 40 years of age, in stable condition.
Criteria for non-inclusion: Restrictive respiratory pathology, Progressive cardiovascular disease, Recent surgery (less than 3 months), All patients who did not pass all the tests in the study protocol were excluded. In addition, participants diagnosed with sleep apnoea syndrome or with a 3% oxygen desaturation index (ODI-3) evaluated by nocturnal oximetry of 15 or more were excluded.
A cross-sectional study design was used. All participants underwent ultrasonographic evaluation, nocturnal oximetry, and other measurements (i.e. pulmonary function). This study was approved by the medical ethics committee of Constantine Regional Military University Hospital, The objective and content of the study were explained verbally as well as in written documents to the participants. Written consent was obtained after the subjects were informed that they could decide whether to participate based on their own free will and that their privacy would be reasonably protected.
Ultrasound measurements of diaphragm
The ultrasound examination was performed by a single specialized and trained professional using a TOSHIBA device, model SSA-370ª Power Vision 6.000.
The right heme diaphragm was examined in B and M mode ultrasound, as it is more easily visualized due to the large acoustic window of the liver. However, the visualization of the left diaphragm is more complex due to the narrower acoustic window of the spleen.
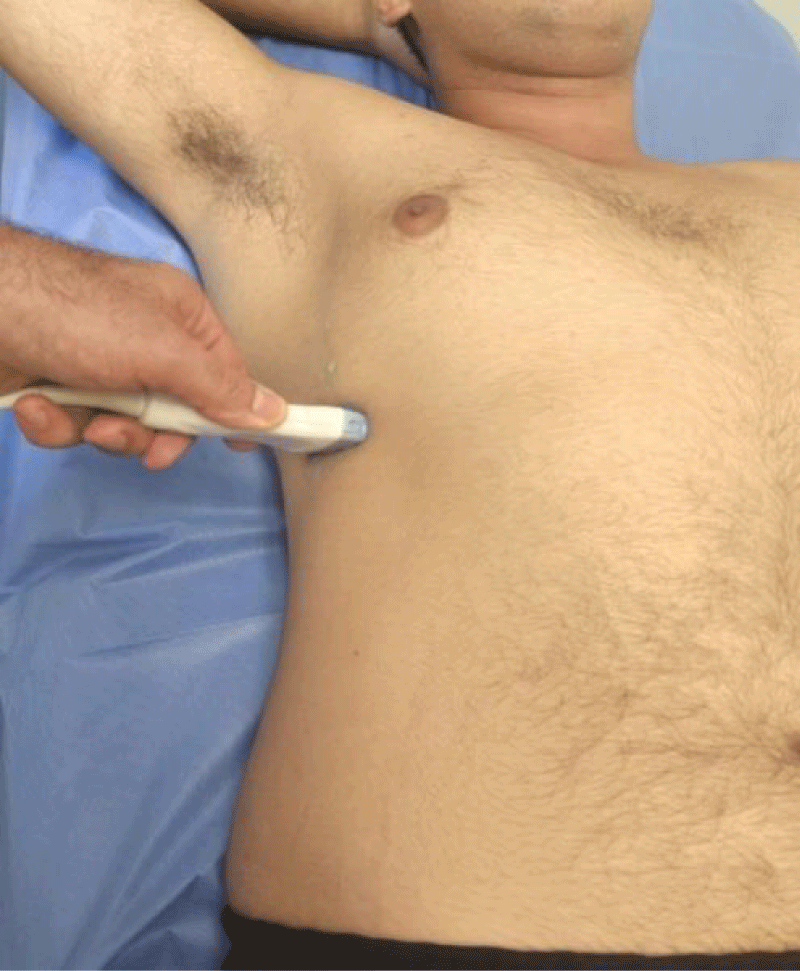
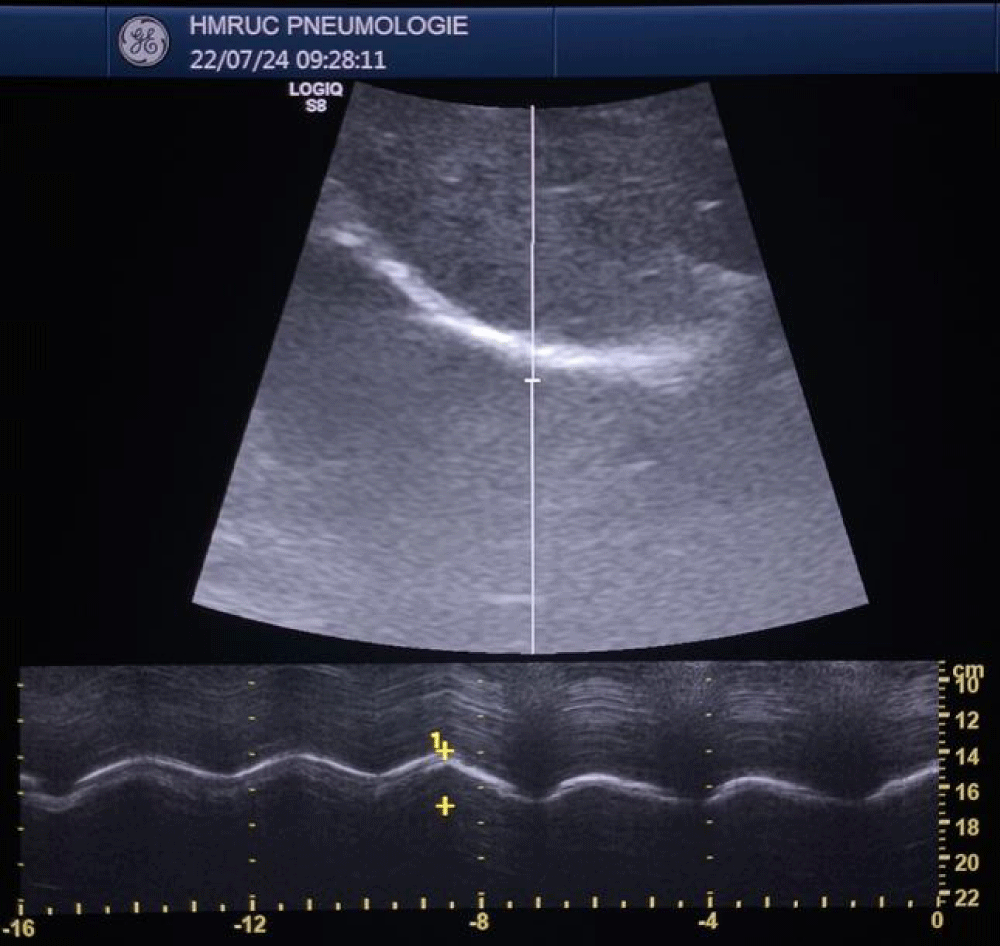
We measured the thickness of the diaphragm (Tfdi) in the zone of apposition using Bmode ultrasound imaging. In the supine position, an 18-5 MHz linear transducer was placed on the chest wall in the eighth or ninth right intercostal space between the anterior-axillary and mid-axillary lines [16,17] (Figure 1). The subject was instructed to take slow deep breaths in and out.
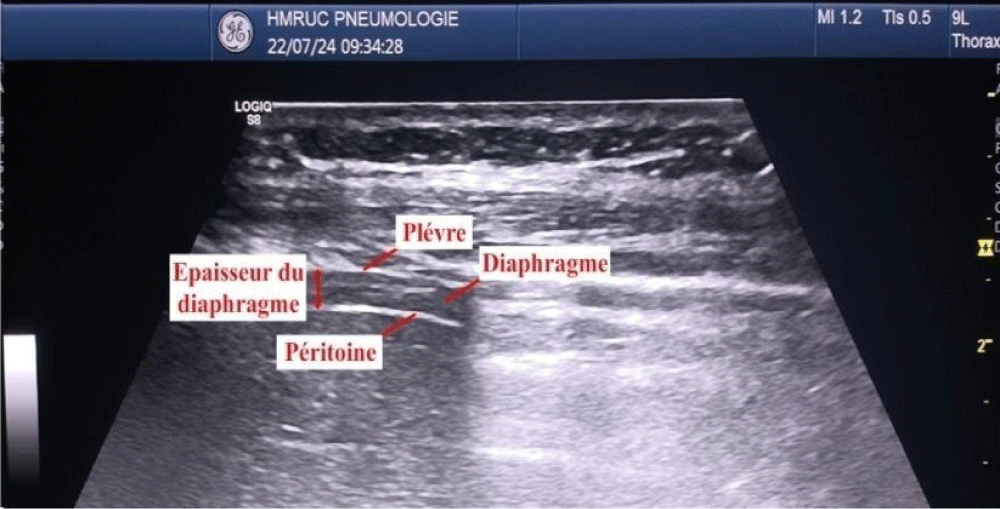
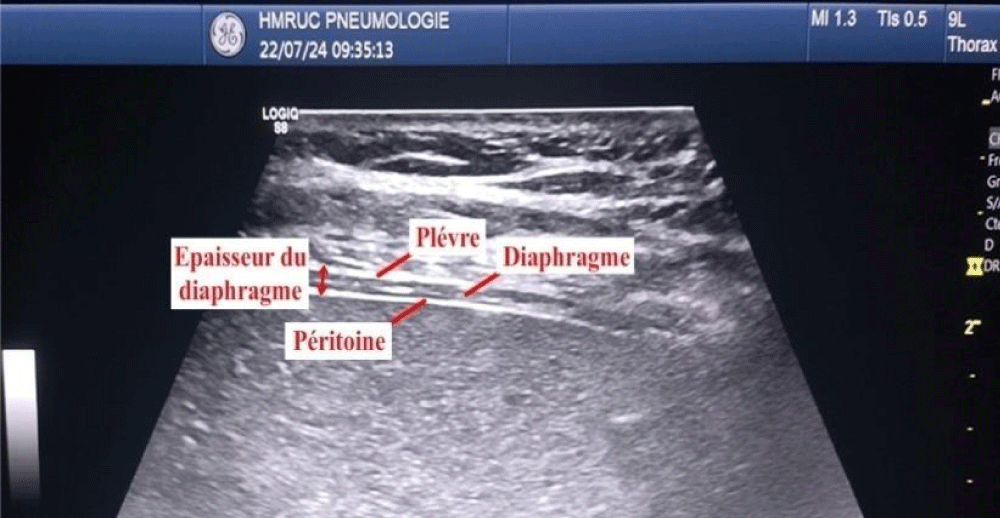
The measure during a breath-holding maneuver at the end of forced expiration (TEE) and at the end of maximal inspiration (TEI). The TFdi was calculated [TEI−TEE)/TEE] (Figures 2,3).
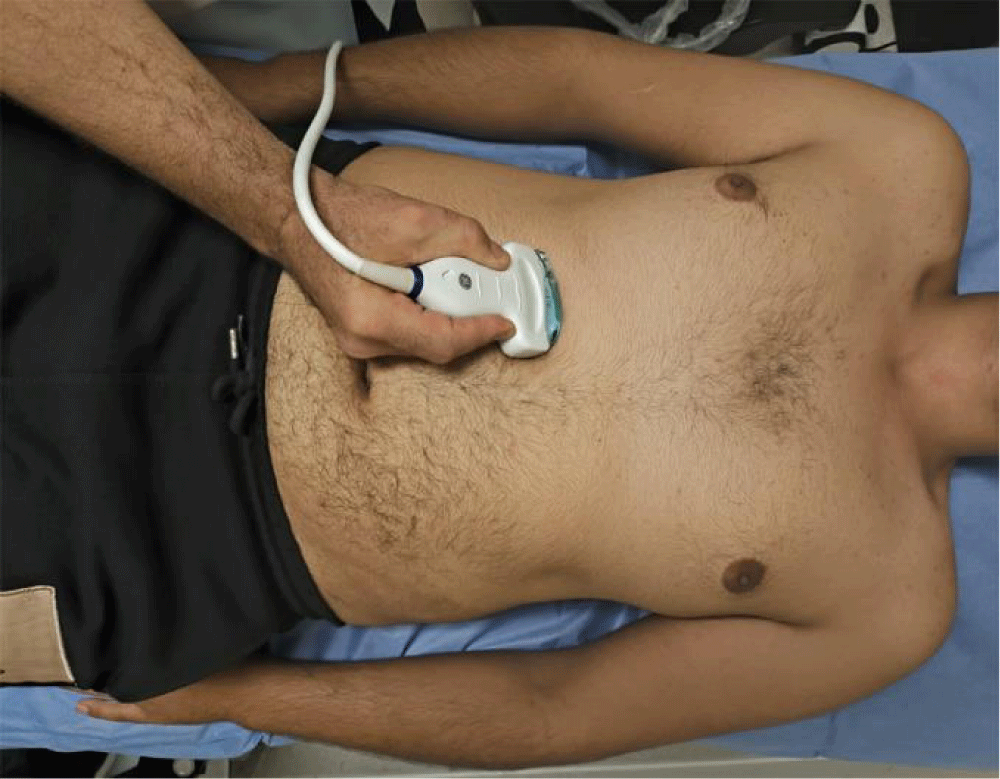
To assess diaphragmatic mobility, (EXdi) the patient was placed in the supine position, and a 3.5 MHz convex transducer was used, which allows the observation of deeper structures. This transducer was positioned in the right subcostal region, in the midclavicular line, with an incidence angle perpendicular to the craniocaudal axis and the diaphragm. This was then identified through the hepatic acoustic window and its mobility was assessed by measuring, in millimeters (through M-mode) its craniocaudal displacement during breathing at rest (tidal volume). Then, the measurement was repeated considering the incursion performed from maximum inspiration (total lung capacity level) to maximum expiration (residual volume level). Three serial measurements were performed, and the highest value was considered and recorded for this research. The transducer was positioned to observe the entire movement of the diaphragmatic excursion, both in the tidal volume maneuver and in the maximal inspiratory and expiratory maneuvers. This technique was previously described and is being widely used [18,19]. All examinations were performed on the right side to take advantage of the acoustic window of the liver, which facilitates visualization of the diaphragm (Figures 4,5).
Nocturnal oximetry
The participants kept a daily sleep log (bedtime and wake-up time) and wore an activity monitor (Actiwatch2, Philips Respironics G.K., Tokyo, Japan) on their non-dominant wrist for overnight oximetry. The time spent in bed (total bedtime, TBT), calculated by their daily sleep log, was used to guide the analysis of the actigraph data recordings. Actigraph data were used to calculate the sleep duration without sleep onset time or snooze time (total sleep time, TST) by analysis software (Actiware, Philips Respironics G.K.).
Overnight domiciliary pulse oximetry was carried out using a pulse oximeter (PULSOX-Me300, Teijin, Ltd., Tokyo, Japan) with a finger probe during TBT. The mean value of the mean of night desaturation during TST was calculated. The nocturnal oxygen desaturation was assessed as the percentage of TST, recorded by actigraphy, with desaturation above 4% (ODI 4%) from the baseline of daytime percutaneous arterial oxygen saturation (SpO2) (SpO2base) for a period of 5 or more minutes.
Other measurements
An arterial blood sample was obtained by puncture of the radial artery for blood gas analysis, namely arterial oxygen pressure (PaO2) and arterial carbon dioxide pressure (PaCO2). Dyspnoea was assessed using the modified Medical Research Council (MMRC) dyspnoea scale.
MMRC dyspnea scale
Degree 0: Patient with dyspnea during intense exercise.
Degree 1: Dyspnea when walking fast on flat ground or climbing a slope light.
Degree 2: Walks slower than people of his age on flat ground, or must
Stop for breath when walking at your own pace on flat ground.
Degree 3: Must stop to breathe after a walk of about 90 meters.
Degree 4: Too breathless to leave home, or dyspnea when dressing.
Statistics analysis
Data was captured and analyzed using IBM SPSS 24.
Many procedures of control at the time of entry have been established to avoid errors as much as possible.
We have implemented the following statistical methods:
- Frequencies and percentages for qualitative data.
- The means, standard deviation, maximum, and minimum for quantitative data.
- The normality of quantitative variables was investigated by the Shapiro-Wilk test.
- Comparisons of summer averages performed by the Student t-test or ANOVA test according to the number of modalities of the variable.
- Comparisons between categorical or nominal variables were made by the Chi2 test or, where appropriate, by the exact Fisher test.
- The study of relationships between quantitative variables was analyzed by the Pearson or Spearman correlation coefficient as a function of the statistical distribution of variables.
The statistical tests used were considered significant when p 0.05 (degree of significance).
Results
The majority of patients included in the study are in both age groups 60 to 70 and 70 to 79, representing 73.8% of the total population (Figure 6), the population in our study is almost exclusively male 1 female/60 male. Sex-ratio = 0.017, the average BMI is 24.12 4.83 kg/m2, with extreme values ranging from 14.6 to 36.9 kg/m2,
Three groups of patients were identified according to smoking status:
- Ex-smokers: They represent the majority of our cohort with 73.8% of patients (n = 45).
- Active smokers: 22.9% of patients are active smokers (n = 14).
- Non-smokers: Only 3.3% of patients have never smoked (n = 2).
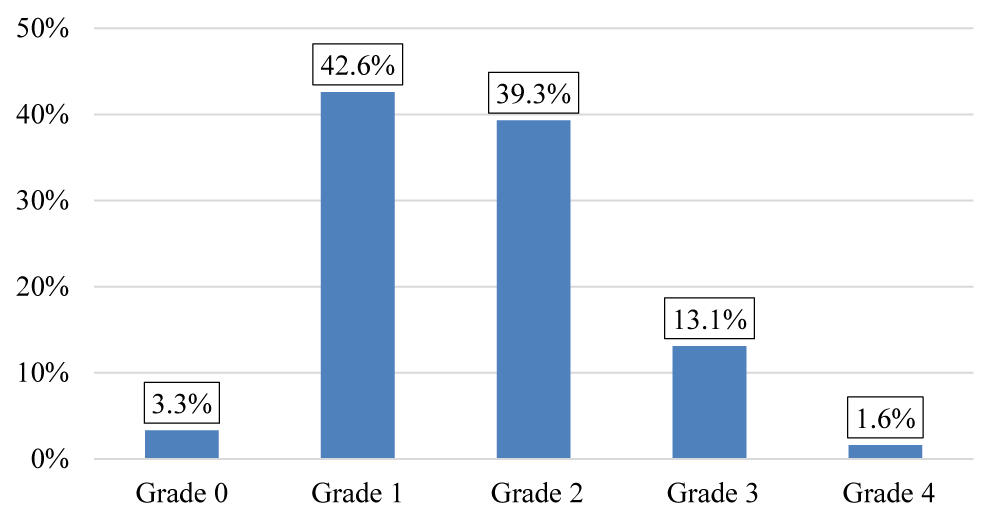
The majority of our patients were classified as grade 1 and grade 2 according to MMRC (Figure 7) the average of PaCO2 is 36,57 (mmHg) (Table 1).
- 62.3% n(38) of patients in our series have a pH between 7.38 and 7.42, 34.4% n(21) have a pH > 7.42 and 3.3% n(2) have a pH < 7.38
- 45.9% n(28) of patients in our series have a PaO2 between 75 and 100 mmHg, 52.5% n(32) have a PaO2 < 75 mmHg
- -85.24% n(52) of the patients in our series have a PaCO2 between 35 and 45 mmHg, 11.47% n(7) have a PaCO2 > 45 mmHg and 3.2% n(2) have a PaCO2 < 35 mmHg
- - 83.6% n(51) of patients in our series have an SaO2 between 94 and 100%, 14.8% n(9) have an SaO2 < 94%
The mean diaphragmatic excursion, evaluated by ultrasound (EXdi), in our patients with COPD, was 5.01 cm ± 1.48 cm, the mean of the thickening fraction, assessed by ultrasound (TFdi), in our patients with COPD was 48.9 % ± 21.1%.
Significant correlations were reported between the echographic and gas-ometric parameters (PaO2, PaCO2), (Tables 2,3).
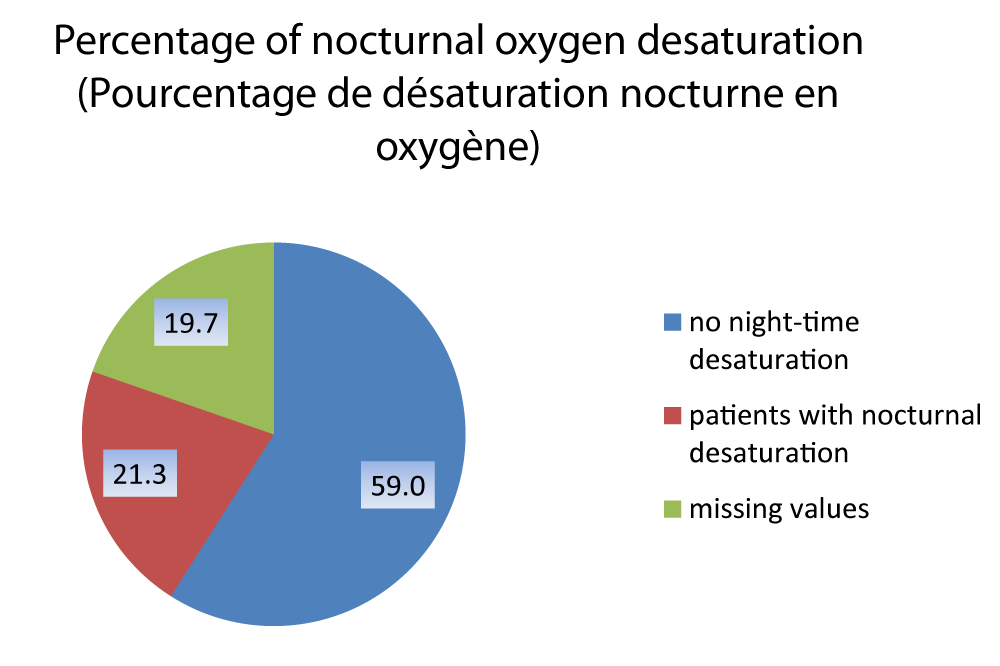
The average nocturnal saturation in our series is 94.63 2.16%, (12 patients could not perform nocturnal oximetry) (Figure 8).
For the 12 patients who did not perform the nocturnal oximetry examination, 08 patients refused hospitalization for the oximetry and 04 failed to examine three successive attempts (during 03 days of hospitalization)
The average ODI 4% (oxygen desaturation index) in our series is 2.01 ± 2.65
Among patients who performed night oximetry n(49), 21.3% between them n(13) have a 4% super ODI greater than 5.
The average night-time saturation was 94,63 ± 2,16% (12 patients could not perform nocturnal oximetry), The average Oxygen Desaturation Index (ODI) was 4% 2,01 ± 2,65, the diaphragmatic excursion (EXdi), with an average of 5.01 1.48 cm in our cohort.
There was a significant positive correlation between diaphragmatic mobility (EXdi) and the average night-time saturation (r = 0,395, p = 0,005). However there was a significant negative correlation between diaphragmatic mobility and the average oxygen desaturation index (ODI 4 %) (r = -0,288, p = 0,045), (Figure 9)
In our study, we found a significant negative correlation between 4% ODI and TFdi (r = - 0, 456 p = 0.001). (Figure 10).
Discussion
In our study, we also observed a significant positive correlation between EXdi and PaO (r = 0.336, p = 0.009), indicating that the higher the PaO2 the better the EXdi. A study by Hafez M, et al. [20], in 2017 on 113 patients with COPD showed similar results, showing that patients with diaphragmatic thinning had significantly lower PaO2 levels compared to those with normal diaphragmatic thickness (t = 5.13, p = 0.001). On the other hand, Amin A, et al. [21] observed a positive, non-significant correlation between EXdi and PaO2 (r = 0.14, p = 0.163), while Kang, Hyun Wook, et al. [22] found no relationship between these parameters (r = 0.028, p = 0.873).
In addition, our results showed that patients with reduced diaphragmatic mobility had higher levels of PaCO2 (r = -0.292, p = 0.024). These observations are in line with those of Bégin, et al. [23] which showed that hypercapnia increases with the severity of obstruction, obesity, and weakness of the inspiratory muscles. Similarly, Scheibe, Nadine, et al. [24] found a moderate correlation with PaCO2 (r = -0.47). ). Kang, Hyun Wook, [22] et al. in a study of 37 patients with stable COPD, also showed significant negative correlations between diaphragmatic mobility and PaCO2 (r = -0.373, p = 0.030), Confirmed by Amin A, et al. [21] (r = -0,45, p = 0,02).
A study conducted in Russia by Volchkov V, et al. [25] in 2016 showed a significant negative correlation between TFdi and PaO2 (r = -0.42, p < 0.05). Contrary to their results, our study did not find a significant correlation between TFDi and PaO2 (r = -0.202, p = 0.118). This may be explained by the difference in severity of COPD between the two groups studied, as Volchkov, et al. included 60 patients with GOLD III and IV COPD, 26 of whom had severe hypoxemia (PaO2 = 53.9 5.9 mmHg), while our study included patients with less advanced stages of COPD.
In the current study, we found that the mean of the TFDi decreased with the increase of PaCO. For a PaCO between 35 and 45 mmHg, the mean of the TFdi was 51.26%, while for a PaCO2 above 45 mmHg, the mean of the TFdi fell to 24.50%. A significant negative correlation was identified between these two parameters (t = -7.26, p = < 0.001). These results are consistent with those of the study by Elsawy, S. B. [26], which showed a progressive decrease in TFdi with the increase of PaCO2 (r = -0.42, p = 0.001).
In addition, Hafez, M. R. and O. I. Abo-Elkheir [20] in 2017 found similar results (r = -0.92, p = 0.001), explaining that diaphragmatic fatigue and muscular strain, combined with airway obstruction and hypercapnia, contribute to diaphragmatic dysfunction in COPD.
In our study, a significant correlation was found between the diaphragmatic excursion (EXdi) and the nocturnal oximetry parameters, including a positive correlation with the mean nocturnal oximetry (r = 0.395, p = 0.005). This suggests that patients with better diaphragmatic mobility also have more stable nocturnal oxygenation levels. In contrast, a negative correlation was observed with the oxygen desaturation index (ODI 4%) (r = -0.288, p = 0.045), which measures the number of drops in oxygen saturation by more than 4% per hour, an indicator frequently used to detect sleep apnea.
These results highlight the importance of diaphragmatic function in managing nocturnal gas exchange in patients with Chronic Obstructive Pulmonary Disease (COPD). Indeed, the EXdi is a key marker of respiratory performance and the diaphragm’s ability to meet ventilatory needs, especially during the REM sleep phase, where diaphragm efficiency becomes crucial to compensate for hypotonia of other respiratory muscles.
Previous studies have shown that nocturnal desaturation is often exacerbated by a reduction in diaphragmatic function, resulting in hypoventilation and hypercapnia at night [27]. These phenomena can be explained by diaphragmatic dysfunction associated with pulmonary hyperinflation, which reduces the effectiveness of the diaphragm by placing it in a flattened and ineffective position. This limits the ability of the diaphragm to generate adequate respiratory movements, resulting in a decrease in nocturnal oxygenation levels, as evidenced by the increase in ODI by 4%.
Nocturnal oxygen desaturation is common in patients with COPD. In our study, a significant negative correlation was observed between the oxygen desaturation index ODI 4% and the TFdi (r = -0.456, p = 0.001). Okura K, et al. [28] also showed that 4% ODI was negatively correlated with TFdi (r = -0.563, p = 0.002) in patients with COPD.
These observations are probably related to hypoventilation associated with a ventilation-perfusion mismatch, particularly marked during REM sleep [29]. During this phase, hypotonia of the postural respiratory muscles, such as the intercostal muscles, reduces the contribution of the thoracic cage to ventilation [30,31]. In COPD patients, this decreased muscle activity aggravates nocturnal hypoventilation, and the diaphragm, although relatively spared by hypotonia, becomes mechanically disadvantaged due to hyperinflation and shortening of muscle fibers [32]. Accumulation of carbon dioxide and loss of contractile proteins further aggravate this dysfunction [33,34].
Conclusion
Ultrasound can assess the activity, function, and force reserve of the diaphragm.
This study highlights the importance of diaphragmatic function in managing nocturnal gas exchange in patients with Chronic Obstructive Pulmonary Disease (COPD).
Nocturnal desaturation is often exacerbated by a reduction in diaphragmatic function, resulting in hypoventilation and hypercapnia during the night.
COPD reduces diaphragm efficiency by placing the diaphragm in a flat and ineffective position. This limits the ability of the diaphragm to generate adequate respiratory movements, resulting in a decrease in nocturnal oxygenation levels, as evidenced by the increase in ODI by 4%.
It is confirmed that diaphragmatic function can be useful in predicting nocturnal oxygen desaturation in patients with COPD.
Authors contribution
A. Meridj: Designed and developed the analysis and wrote the article; R. Belala: Collected data; K. Tlili: Contributed to data or analytics tools; Y. Djeghri: Conducted the analysis.
Our gratitude to Professor Djeghri Yacine who supported this work.
Thank you to all the authors who have contributed to the literature review, critically reviewed the manuscript for its important intellectual content, approved the final version to be published, and agreed to act as guarantors of the work.
This study was performed in accordance with the Declaration of Helsinki. This human study was approved by the Comié d’ethique of Regional Military University Hospital. All adult participants provided written informed consent to participate in this study.
All authors report not using Artificial Intelligence (AI) assisted technologies (such as Large Language Models [LLM], chatbots, or image creators) in the production of submitted work.
- Agustí A, Celli BR, Criner GJ, Halpin D, Anzueto A, Barnes P, et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Am J Respir Crit Care Med. 2023;207(7):819-837. Available from: https://doi.org/10.1164/rccm.202301-0106pp
- Chabot F, Zysman M, Guillaumot A, Gomez E, Kheir A, Chaouat A. Chronic obstructive pulmonary disease. Bull Acad Natl Med. 2019;203(1-2):63-71. Available from: https://doi.org/10.1016/j.banm.2019.03.007
- Raherison C, Girodet PO. Epidemiology of COPD. Eur Respir Rev. 2009;18(114):213-221. Available from: https://doi.org/10.1183/09059180.00003609
- Adeloye D, Chua S, Lee C, Basquill C, Papana A, Theodoratou E, et al. Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J Glob Health. 2015;5(2):020415. Available from: https://doi.org/10.7189/jogh.05.020415
- Buist A, Vollmer W, McBurnie M. Worldwide burden of COPD in high-and low-income countries. Part I. The Burden of Obstructive Lung Disease (BOLD) Initiative. Int J Tuberc Lung Dis. 2008;12(7):703-8. Available from: https://pubmed.ncbi.nlm.nih.gov/18544191/
- Khelafi R, Aissanou A, Tarsift S, Skander F. Épidémiologie de la bronchopneumopathie chronique obstructive dans la wilaya d’Alger. Rev Mal Respir. 2011;28(1):32-40. Available from: https://doi.org/10.1016/j.rmr.2010.06.026
- Rennard S, Decramer M, Calverley P, Pride N, Soriano J, Vermeire P, et al. Impact of COPD in North America and Europe in 2000: subjects' perspective of Confronting COPD International Survey. Eur Respir J. 2002;20(4):799-805. Available from: https://doi.org/10.1183/09031936.02.03242002
- Ding B, Small M, Bergström G, Holmgren U. A cross-sectional survey of night-time symptoms and impact of sleep disturbance on symptoms and health status in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017:589-599. Available from: https://doi.org/10.2147/copd.s122485
- Greening NJ, Harvey-Dunstan TC, Chaplin EJ, Vincent EE, Morgan MD, Singh SJ, et al. Bedside assessment of quadriceps muscle by ultrasound after admission for acute exacerbations of chronic respiratory disease. Am J Respir Crit Care Med. 2015;192(7):810-816. Available from: https://doi.org/10.1164/rccm.201503-0535oc
- Lin CL, Yeh C, Yen CW, Hsu WH, Hang LW. Comparison of the indices of oxyhemoglobin saturation by pulse oximetry in obstructive sleep apnea-hypopnea syndrome. Chest. 2009;135(1):86-93. Available from: https://doi.org/10.1378/chest.08-0057
- Vazquez J, Tsai WH, Flemons WW, Masuda A, Brant R, Hajduk E, Whitelaw WA, Remmers JE. Automated analysis of digital oximetry in the diagnosis of obstructive sleep apnoea. Thorax. 2000;55:302-307. Available from: https://doi.org/10.1136/thorax.55.4.302
- Varghese L, Rebekah G, Oliver A, Kurien R. Oxygen desaturation index as alternative parameter in screening patients with severe obstructive sleep apnea. Sleep Sci. 2022;15(S 01):224-228. Available from: https://doi.org/10.5935/1984-0063.20200119
- Chung F, Liao P, Elsaid H, Islam S, Shapiro CM, Sun Y. Oxygen desaturation index from nocturnal oximetry: a sensitive and specific tool to detect sleep-disordered breathing in surgical patients. Anesth Analg. 2012;114(5):993-1000. Available from: https://doi.org/10.1213/ane.0b013e318248f4f5
- Meridj A, Belaala R, Djeghri Y. Influence of COPD on the diaphragm and muscles of the lower limbs. J Pulmonol Respir Res. 2024;8(2):056-059. Available from: https://doi.org/10.29328/journal.jprr.1001061
- Heijdra Y, Dekhuijzen P, Van Herwaarden C, Folgering H. Nocturnal saturation and respiratory muscle function in patients with chronic obstructive pulmonary disease. Thorax. 1995;50(6):610-612. Available from: https://doi.org/10.1136/thx.50.6.610
- McCool FD, Benditt JO, Conomos P, Anderson L, Sherman CB, Hoppin Jr FG. Variability of diaphragm structure among healthy individuals. Am J Respir Crit Care Med. 1997;155(4):1323-1328. Available from: https://doi.org/10.1164/ajrccm.155.4.9105074
- Boon AJ, Harper CJ, Ghahfarokhi LS, Strommen JA, Watson JC, Sorenson EJ. Two-dimensional ultrasound imaging of the diaphragm: quantitative values in normal subjects. Muscle Nerve. 2013;47(6):884-889. Available from: https://doi.org/10.1002/mus.23702
- Vivier E, Mekontso Dessap A, Dimassi S, Vargas F, Lyazidi A, Thille AW, et al. Diaphragm ultrasonography to estimate the work of breathing during non-invasive ventilation. Intensive Care Med. 2012;38:796-803. Available from: https://doi.org/10.1007/s00134-012-2547-7
- Schulz A, Erbuth A, Boyko M, Vonderbank S, Gürleyen H, Gibis N, et al. Comparison of ultrasound measurements for diaphragmatic mobility, diaphragmatic thickness, and diaphragm thickening fraction with each other and with lung function in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2022:2217-27. Available from: https://doi.org/10.2147/copd.s375956
- Hafez MR, Abo-Elkheir OI. Sonographic assessment of diaphragm thickness and its effect on inspiratory muscles' strength in patients with chronic obstructive pulmonary disease. Eurasian J Pulmonol. 2017;19(2). Available from: http://dx.doi.org/10.5152/ejp.2017.42104
- Amin A, Zedan M. Transthoracic ultrasonographic evaluation of diaphragmatic excursion in patients with chronic obstructive pulmonary disease. Egypt J Bronchol. 2018;12:27-32. Available from: https://ejb.springeropen.com/articles/10.4103/1687-8426.217411
- Kang HW, Kim TO, Lee BR, Yu JY, Chi SY, Ban HJ, et al. Influence of diaphragmatic mobility on hypercapnia in patients with chronic obstructive pulmonary disease. J Korean Med Sci. 2011;26(9):1209-1213. Available from: https://doi.org/10.3346/jkms.2011.26.9.1209
- Bégin P, Grassino A. Inspiratory muscle dysfunction and chronic hypercapnia in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1991;143(5 Pt 1):905-912. Available from: https://doi.org/10.1164/ajrccm/143.5_pt_1.905
- Scheibe N, Sosnowski N, Pinkhasik A, Vonderbank S, Bastian A. Sonographic evaluation of diaphragmatic dysfunction in COPD patients. Int J Chron Obstruct Pulmon Dis. 2015:1925-1930. Available from: https://doi.org/10.2147/copd.s85659
- Volchkov V, Titova O, Sklyarova D, Kusubova N. The functional state of the diaphragm in COPD patients with hypoxemia. Eur Respir Soc. 2016. Available from: https://channel.ersnet.org/media-34510-the-functional-state-of-the-diaphragm-in-copd-patients-with-hypoxemia
- Elsawy SB. Impact of chronic obstructive pulmonary disease severity on diaphragm muscle thickness. Egypt J Chest Dis Tuberc. 2017;66(4):587-92. Available from: http://dx.doi.org/10.1016/j.ejcdt.2017.08.002
- Spiesshoefer J, Lutter R, Kabitz HJ, Henke C, Herkenrath S, Randerath W, et al. Respiratory muscle function tests and diaphragm ultrasound predict nocturnal hypoventilation in slowly progressive myopathies. Front Neurol. 2021;12:731865. Available from: https://doi.org/10.3389/fneur.2021.731865
- Okura K, Kawagoshi A, Iwakura M, Sugawara K, Takahashi H, Kashiwagura T, et al. Contractile capability of the diaphragm assessed by ultrasonography predicts nocturnal oxygen saturation in COPD. Respirology (Carlton, Vic). 2017;22(2):301-306. Available from: https://doi.org/10.1111/resp.12897
- Fletcher EC, Gray BA, Levin DC. Nonapneic mechanisms of arterial oxygen desaturation during rapid-eye-movement sleep. J Appl Physiol. 1983;54(3):632-639. Available from: https://doi.org/10.1152/jappl.1983.54.3.632
- Johnson MW, Remmers JE. Accessory muscle activity during sleep in chronic obstructive pulmonary disease. J Appl Physiol. 1984;57(4):1011-1017. Available from: https://doi.org/10.1152/jappl.1984.57.4.1011
- Millman RP, Knight H, Kline LR, Shore ET, Chung D, Pack AI. Changes in compartmental ventilation in association with eye movements during REM sleep. J Appl Physiol. 1988;65(3):1196-1202. Available from: https://doi.org/10.1152/jappl.1988.65.3.1196
- Phillipson EA. Control of breathing during sleep. Am Rev Respir Dis. 1978;118(5):909-939. Available from: https://pubmed.ncbi.nlm.nih.gov/216294/
- Ottenheijm CA, Heunks LM, Sieck GC, Zhan W-Z, Jansen SM, Degens H, et al. Diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(2):200-205. Available from: https://doi.org/10.1164/rccm.200502-262oc
- Similowski T, Yan S, Gauthier AP, Macklem PT, Bellemare F. Contractile properties of the human diaphragm during chronic hyperinflation. N Engl J Med. 1991;325(13):917-923. Available from: https://doi.org/10.1056/nejm199109263251304
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
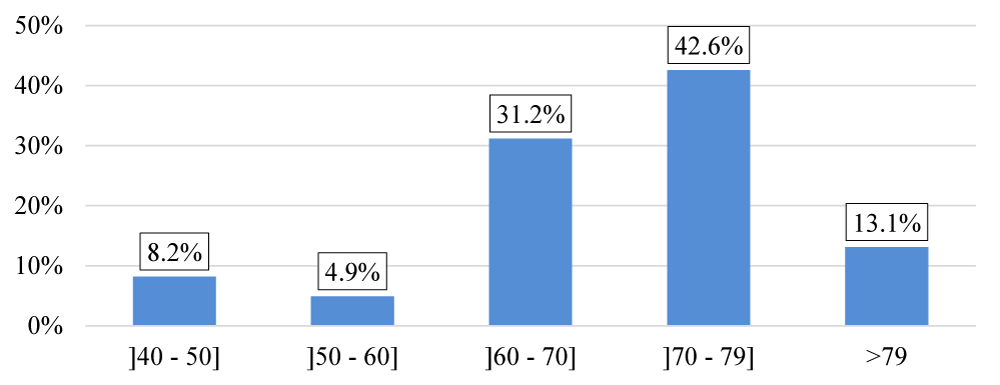


 Save to Mendeley
Save to Mendeley
