Archives of Pulmonology and Respiratory Care
The Clinical Impact of the Film Array (Biofire) Respiratory Panel Utilization on the Outcomes of Pediatric Patients with Acute Viral Respiratory Infections in Two Private Tertiary Hospitals in Cebu
1Chong Hua Hospital Mandaue, Cebu, Philippines
2Chong Hua Hospital, Cebu, Philippines
Author and article information
Cite this as
Julasiri S, Lim J, Kimseng KJ. The Clinical Impact of the Film Array (Biofire) Respiratory Panel Utilization on the Outcomes of Pediatric Patients with Acute Viral Respiratory Infections in Two Private Tertiary Hospitals in Cebu. Arch Pulmonol Respir Care. 2025; 11(1): 001-010. Available from: 10.17352/aprc.000091
Copyright License
© 2025 Julasiri S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: Acute Respiratory tract Infections (ARI) are the most prevalent illness in people of all ages, and they are a leading cause of hospitalization and death. Molecular testing methods have significantly expanded the ability to diagnose respiratory infections. Rapid viral testing aims to prompt the diagnosis of viral infections that could lead to faster hospital discharge, and lower healthcare resource use, and clinicians are guided on the judicious use of antibiotics, as well as greater isolation precautions. The objective of this study was to determine the clinical impact of the Film Array (Biofire) Respiratory Panel utilization on the outcomes of pediatric patients with acute viral respiratory infection.
Methodology: This is a cross-sectional analytic study, conducted in two private tertiary hospitals. The study population includes admitted patients aged 1-18 years old with an acute respiratory infection and then divided into two groups: exposure group (with Biofire taken) and non-exposure group (without Biofire taken). Retrospective chart review was done on the admitted patients and analyzed using descriptive and inferential statistics.
Results: A total of 220 samples were included. The majority of patients in both groups were female, aged 1-5 years old, lived in an urban locality, and with no influenza vaccination. The most common virus detected was hRV/hEV(n = 29%), while the most common codetection virus was hRV/hEV with influenza B (n = 23.5%). For those who underwent the test, patients were frequently admitted in the year 2021 (n = 90%) and the month of July (n = 28.2%). Utilization of the respiratory panel was associated with significant changes in medical management including decreased antibiotic usage (p = 0.001) and shorter length of hospital stay (p = 0.029), compared to those patients who didn’t undergo the test.
Conclusion: The Film Array (Biofire) respiratory panel is useful in assisting clinical judgment regarding the usage of antibiotics as well as the length of hospitalization among children affected by acute respiratory infections.
Background of the study
Acute Respiratory tract Infections (ARI) are the most prevalent illness in people of all ages, and they are a leading cause of hospitalization and death [1]. Clinical symptoms, laboratory evaluations, and imaging are frequently insufficient to identify the underlying pathogen [2]. The common cold, otitis media, pharyngitis, acute bronchiolitis, and pneumonia are the most prevalent respiratory illnesses among individuals seeking medical care accounting for the bulk of antibiotic prescriptions [3]. ARIs are also responsible for children’s high antibiotic use, even though the majority of ARIs are viral [4]. In pediatric outpatients, viral acute respiratory infections place a tremendous strain on emergency departments and patients’ families [5]. Molecular testing methods have significantly expanded the ability to diagnose respiratory infections [6]. The Film Array Respiratory Panel was initially introduced in 2011 to detect respiratory viruses. In 2012, it was upgraded to include four bacterial strains and 19 viruses with an overall sensitivity and specificity of 97.4% and 99.4%, respectively [7,8]. Several studies have shown that identifying a specific respiratory pathogen can improve a patient’s chances of being discharged successfully [9]. These studies also noted that the BioFire Film Array did not affect the length of stay in a hospital [10]. Specific viral pathogens are promptly identified, and clinicians are guided on the judicious use of antibiotics, which in turn will prevent the development of antibiotic resistance [11].
Significance of the study
By establishing the advantages of utilizing the Biofire respiratory panel to determine the specific viral etiology of acute respiratory infections, physicians may be able to prevent unnecessary antibiotic administration, promote cost-effective treatment techniques, improve clinical decision-making abilities, increase the accuracy of patient diagnoses, decrease hospital stays, and improve resource allocation.
Research question
Among pediatric patients with acute viral respiratory infections in two tertiary private hospitals, what is the clinical impact of the utilization of the Film Array (Biofire) respiratory panel on their outcomes?
Review of literature
Acute respiratory infections (ARI) are the most common illness among people of all ages and genders [1]. The clinical signs of respiratory tract infections frequently do not correlate with the bacteria that is causing the infection. However, distinguishing between bacterial and viral causes is critical for effective treatment [2]. Antibiotic-resistant microbial infections are associated with increased morbidity, mortality, and a significant economic burden [3]. Viral infections can be diagnosed using a variety of methods. Polymerase Chain Reaction (PCR)-based assays have recently been introduced and numerous studies have demonstrated that panels that can identify a wide range of viral infections significantly improve diagnostic output [4]. Acute viral respiratory infections in pediatric outpatients are a huge burden on emergency departments and patients’ families, especially during influenza seasons, accounting for roughly 20% of all deaths in preschool children worldwide, with pneumonia accounting for 90% of these deaths [5]. Past epidemiologic studies used routine diagnostic methods such as culture to detect viral and bacterial respiratory pathogens [6]. Molecular Respiratory Panel (MRP) assays are gaining popularity as a result of their ability to detect numerous diseases with excellent sensitivity and specificity as well as demonstrated cost reductions [12].
The BIOFIRE® Respiratory 2.1 plus Panel tests for 19 viruses and four bacteria cause respiratory tract infections with an overall sensitivity and specificity of 97.4% and 99.4%, respectively [8]. It integrates sample preparation, amplification, detection, and analysis into one simple system that requires just 2 minutes of hands-on time, with a total run time of about an hour [8].
In developed countries, acute respiratory tract infection is the most common cause of hospitalization of children. A rapid and accurate diagnosis such as PCR testing is required to start adequate therapy, minimize hospital admission, and reduce unnecessary antibiotic use [13].
Even when viral infection is a strong possibility, empiric antibiotic treatment is frequently started, leading to unnecessary antibiotic use. Reducing the use of antibiotics affects reducing side effects and aids in efforts by the public health sector to address rising antibiotic resistance [14-16]. In a retrospective study by Rogers, et al. they stated that healthcare providers may be more confident in discharge after they have identified a specific pathogen associated with the patient’s illness [10]. One such study by Andrews, et al. performed in adult patients compared the BioFire Film Array to routine laboratory-based PCR and serology tests and found no evidence of an association between Film Array testing and length of hospital stay. They attributed their length of stay results to a delay in initiation of the Film Array nasal swab by clinical staff due to the lack of hospital procedures [11]. Although the introduction of real-time polymerase chain reaction testing into routine clinical practice has resulted in a large number of viral diagnoses. It has had little impact on patient treatment [17]. However, in a study conducted by Subramony, et al. (2016). use of multiplex polymerase chain reaction testing for respiratory viruses among hospitalized patients was significantly associated with decreased healthcare resource utilization including the use of antibiotics, chest radiographs and increased use of isolation precautions [18].
In a study conducted by Lee, et al. (2018), they demonstrated that adopting MRP assays with a quicker turnaround time can result in significant improvements for pediatric inpatients with viral acute respiratory tract infections [19]. But in a study conducted by Brendish, et al. (2017), they concluded that routine molecular point-of-care testing for respiratory viruses in adults secondary care with acute respiratory illness improved turnaround time result in detection rate but did not reduce the proportion of patients treated with antibiotic or the overall duration of antibiotic use. Therefore, rapid diagnostics can assist physicians in making wise decisions on the use of antibiotics for patients with acute respiratory tract infections by promptly delivering respiratory pathogen data [19,20].
General objective: The objective of this study was to determine the clinical impact of the Film Array (Biofire) Respiratory Panel utilization on the outcomes of pediatric patients with acute viral respiratory infection
Specific objectives:
1. To describe the admitted pediatric patients with acute viral respiratory infection in terms of the following demographic variables:
- Age
- Sex
- Month and Year Admitted
- Influenza vaccination status
- Residence (Urban vs. Rural)
2. To determine the distribution of specific pediatric viral respiratory pathogens based on:
- Age group
- Month and Year
- Influenza vaccination status
- Residence (Urban vs. Rural)
3. To determine how the use of the Film Array (Biofire) respiratory panel test among hospitalized pediatric patients with viral respiratory infections affected the following:
- Admission type (Noncritical vs. Critical care unit)
- Antibiotic Usage
- Length of Hospital stay
- Status on discharge (Improved, With Oxygen dependence or Sequelae, and Expired)
Methodology
Study design
The researcher used a cross-sectional analytic study design, using a retrospective chart review of pediatric patients admitted from October 2018 to March 2022.
Study setting
The study was conducted in two tertiary private hospitals in Cebu that are both capable of performing Film Array (Biofire) respiratory panels.
Study population
The subjects were divided into an exposure group (with Biofire Respiratory panel) and a non-exposure group (without Biofire respiratory panel)
Inclusion criteria
Pediatric patients ages 1 month to 18 years old, diagnosed with acute viral respiratory infection, admitted in either two private tertiary hospitals in Cebu and Mandaue from October 2018 to March 2022 and with negative culture growth in any specimen ( Blood, CSF, Throat, Tracheal Aspirate, Urine, Wound).
Exclusion criteria
Admitted more than once in the same year in either two private tertiary hospitals in Cebu and Mandaue, discharged against medical advice (DAMA) or transferred to another institution, immunocompromised patient or receiving immunosuppressive therapy, with respiratory and non-respiratory comorbidities, Bacteria identified in the Film Array (Biofire) respiratory panel 2 or 2.1 and with positive culture growth in any specimen (Blood, CSF, Throat, Tracheal Aspirate, Urine, Wound)
Limitation of the study
The data gathered is limited to the information provided by the medical records. Three and half years of chart review is available since the respiratory film array (Biofire) was only utilized in the last quarter of 2018. Furthermore, the study population only included admitted patients for better monitoring of the course of the illness. Hence, the overall severity of the respiratory illness reflected in the study may be worse than that of the general population during the study period.
Sample size
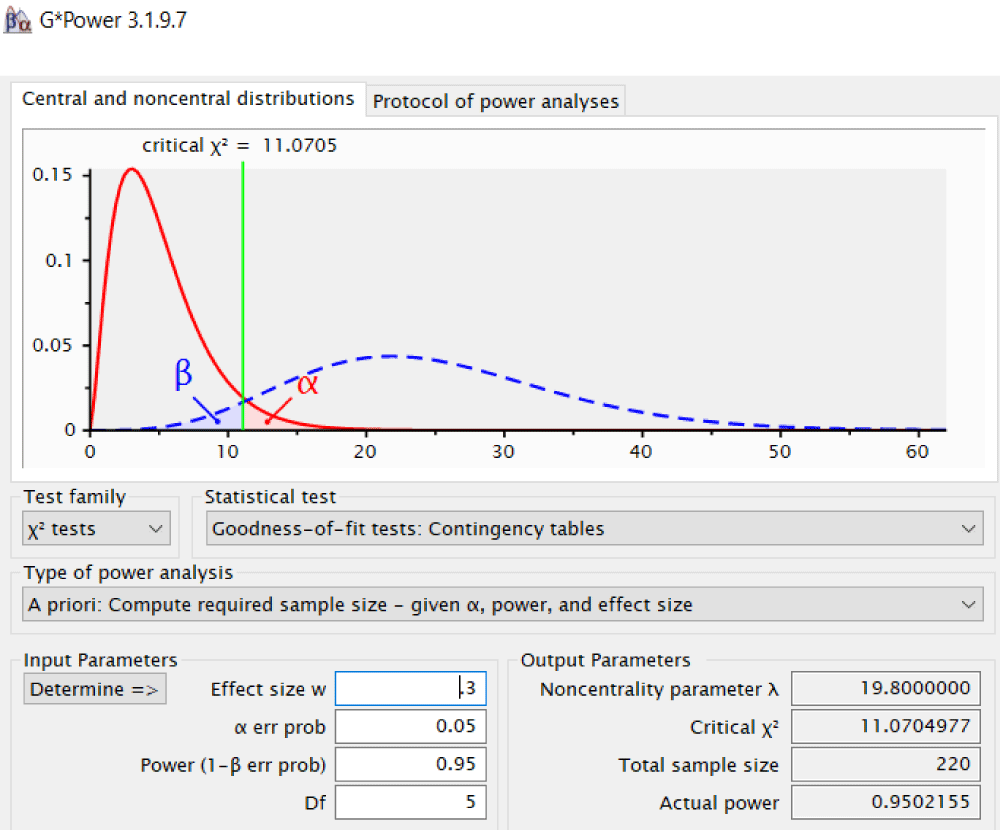
Purposive sampling was used in this investigation. In computing the sample size for the chi-square goodness of fit test (contingency tables), the GPower version 3.19.7 was used (Figure 1). The study had a total sample size estimate of 220 participants which was subdivided into 110 participants for the non-exposure group and 110 for the exposure group. The first 110 participants who fulfilled the inclusion and exclusion criteria on both exposure and non-exposure groups starting from March 2022 down to October 2018 were included in the study.
Data collection and analysis
Once the study was approved by the institutional review board, a letter was sent to the main laboratory to seek permission to retrieve the complete master list of the patients who underwent the Biofire Respiratory Panel 2 or 2.1. Likewise, a letter to the head of the records section was sent to ask permission to review the charts of pediatric (1-month to 18-year-old) patients diagnosed with an acute viral respiratory infection. Once approved to collect data, the researcher identified the exposure group and the non-exposure group. The patient’s demographic data (age, sex, month and year admitted, influenza vaccine status, residence, admission type, antibiotic usage, length of hospital stay and status on discharge) and distribution of specific pathogens detected in Film Array (Biofire) respiratory panel were collected. These data were encoded in a master table in Microsoft Excel. Analysis of the results was conducted based on the objectives of this study. Quantitative descriptive statistics were used to describe and summarize the dataset obtained in the study.
The chi-square test of independence was conducted to determine the association between admission type, antibiotic usage, length of hospital, and status in discharge as outcomes of the usage of the Film Array (Biofire) Respiratory panel and those who didn’t undergo the test.
Ethical considerations
After securing approval from the Chong Hua Hospitals’ Institutional Review Board (IRB) with IRB reference code IRBi-9121-10, the study commenced. The primary investigator acknowledged that the study population consists of vulnerable pediatric patients. Due diligence in upholding the subject’s rights including privacy and patient confidentiality was implemented. Informed consent was obtained from the parents and/or legal guardians for the study. The data were collected through a review of charts and the anonymity of the subject identifiers throughout the study was ensured. Any electronically encoded study participant information was password protected with access only to the study investigator. All soft copies will be deleted and hard copies will be shredded after five years from the time of submission to Chong Hua Hospital IRB.
Results and interpretation of data
A total of 220 participants were included and analyzed in this study. The results are reflected as follows:
The majority of the pediatric patients with acute viral respiratory infections are 1-5 years old from both the exposure group (n = 77, 70.0%) and non-exposure group (n = 70, 63.6%) as shown in Table 1 below.
In terms of gender, there are more females than males for both the exposure group (n = 71, 64.5%) and the non-exposure group (n = 66, 60.0%) as reflected in Table 2 below.
Table 3 below shows In terms of the distribution of admitted patients per month with acute viral respiratory infection from October 2018 to March 2022, the majority of the pediatric patients with acute viral respiratory infections were admitted in July (n = 31, 28.2%) for the exposed group. While the majority of the non-exposed group was admitted in October (n = 34, 30.9%).
Many of the respondents under the exposed group were admitted in the year 2021 (n = 100, 90.0%). On one hand, most of the respondents in the non-exposure group were admitted in the year 2018 (n = 47 42.7%) as shown in Table 4 below.
As shown in Table 5 below is the distribution of the ARI participants per locality. The majority of the sampled pediatric patients for both the exposure group (n = 92, 83.6%) and the non-exposure group (n = 75, 68.2%) are residing in an urban locality.
In terms of influenza vaccination status, a greater number of sampled pediatric patients for both the exposure group (n = 81, 73.6%) and non-exposure group (n = 90, 81.8%) are not vaccinated as shown in Table 6.
For single viral pathogen detected, human rhinovirus/enterovirus had the highest frequency (n = 27, 29%), while Coronavirus HKU1 (n = 1, 1.1%) and Parainfluenza virus (n = 1, 1.1%) had the lowest frequency. For viral pathogens that are co-detected with others, human rhinovirus/enterovirus co-detected with influenza B had the highest frequency (n = 4, 23.5%) as shown in Table 7.
These are the viral pathogens that are frequently detected among the sampled pediatric patients by age group. For the patients whose age is less than a year, only the viral pathogen coronavirus HKU 1 was detected. While the rest of the single viral pathogens were frequently detected among the 1-5 years old age group. For co-detected viral pathogens, they are more frequently detected between the ages of 1-5 years old (n = 13, 76.5%) as reflected in Table 8 below.
In terms of gender, Coronavirus HKU 1 was detected in one female pediatric patient. The frequency of detection for Human Metapneumovirus and Parainfluenza Virus 3 viral pathogens are equal among male and female pediatric patients. While the rest of the single-detected viruses are more frequent among male pediatric patients. For co-detected viral pathogens, it is more frequently detected among female pediatric patients than among males as shown in Table 9 below.
For the distribution of single pathogen detection by month as shown in the Table 10 below, Coronavirus HKU 1 and Parainfluenza Virus 1 were noted to be the single pathogens detected for January (n = 1, 100%) and July (n = 1, 11%), respectively. Human Metapneumovirus was most frequently detected in October (n = 4, 28.6%) and November (n = 4, 28.6%) while Human Rhinovirus/Enterovirus was most frequently detected in October (n = 6, 22.2%). The viral pathogen Influenza A was most frequently detected in November (n = 3, 60%) while Influenza B was most frequently detected in July (n = 18, 81.8%). The majority of the cases of pediatric patients detected with Parainfluenza Virus 3 are in May (n = 2, 50%). Respiratory Syncytial Virus was detected in patients admitted in November (n = 3, 37.5%) while detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2 was common in August (n = 4, 44.4%) and September (n = 4, 44.4%). Furthermore, viral pathogens with codetection were commonly detected in July (n = 3, 17.6%) and December (n = 3, 17.6%).
In terms of the year admitted, all the viral pathogens, including codetection, were commonly detected in the year 2021, except for Coronavirus HKU 1, wherein the single case was in the year 2022 as shown in Table 11 below.
In terms of locality, all the viral pathogens, including codetection, were more frequently detected among pediatric patients living in urban areas, except for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) which was more frequently detected among pediatric patients living in a rural locality (n = 8, 88.9%) as shown in Table 12 below.
In terms of influenza vaccination status, the viral pathogens Coronavirus HKU 1, Human Metapneumovirus, Human Rhinovirus/Enterovirus, Influenza A, Parainfluenza Virus 1, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) were more frequently detected among pediatric patients who were non-vaccinated than those who had vaccination. On the other hand, the viral pathogens Influenza B, and Respiratory Syncytial Virus were more frequently detected among pediatric patients who had influenza vaccination relative to those who were not vaccinated. The frequency of detection among vaccinated versus unvaccinated is equal for the viral pathogen Parainfluenza Virus 3. Viral pathogens with codetection are more frequently detected among non-vaccinated pediatric patients as reflected in Table 13 below.
The outcomes among hospitalized pediatric patients, who underwent the Film Array (Biofire) respiratory panel were analyzed. For admission type and status on discharge, it was not subjected to statistical treatment because all the data gathered regarding admission type were in the majority, pediatric patients admitted in non-critical set-up while for status on discharge, all data sampled were pediatric patients with “improved” status when they were discharged. No long-term sequelae or mortality were documented in these pediatric patients.
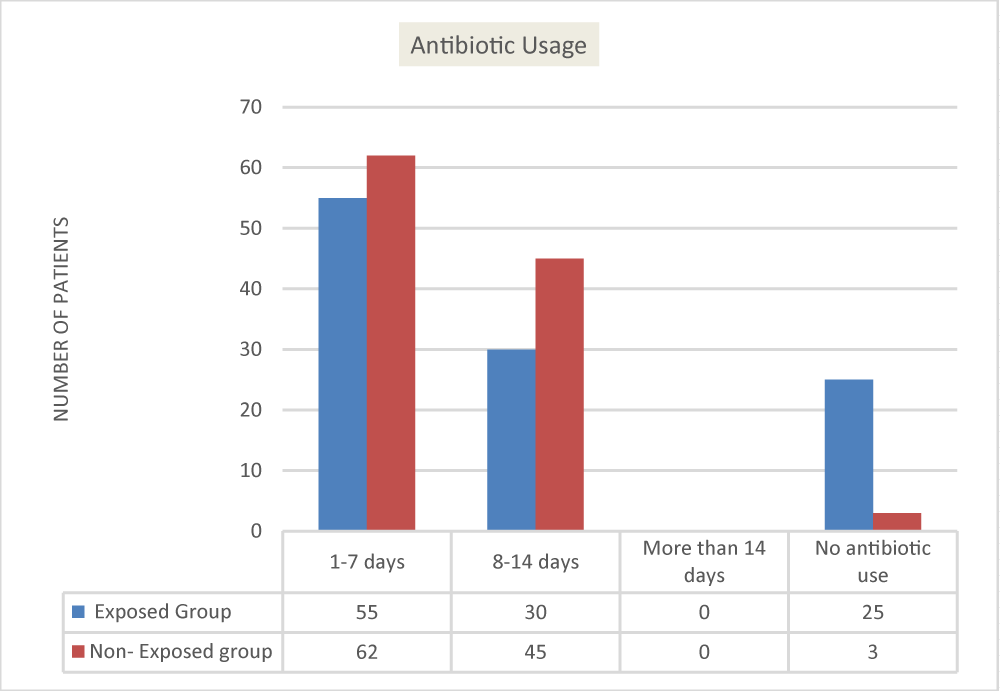
The bar graph below (Figure 2) contains the data bearing on the contingency between the kind of group (exposure group versus non-exposure group) and antibiotic usage (1-7 days, 8-14 days, no antibiotic use). Examining the pattern of the frequencies, it can be noted that there is a greater frequency of pediatric patients under the non-exposure group who had antibiotic usage for 1-7 days and 8-14 days than pediatric patients under the exposure group (utilized Biofire). On one hand, there is a greater frequency of pediatric patients under the exposure group (utilized Biofire) who had no antibiotic use relative to the pediatric patients under the non-exposure group.
The chi-square results show that the type of group differs significantly in terms of antibiotic usage (x2 = 20.71, p =.001). The p - value (two-tailed) is smaller than the standard alpha value of 0.05, so the null hypothesis that the conditions are independent of each other should be rejected. This means that there is a significant association in antibiotic use (no usage of antibioti) between pediatric patients, who underwent Film Array (Biofire) respiratory panel (exposure group) versus pediatric patients, who did not undergo Film Array (Biofire) respiratory panel (non-exposure group).
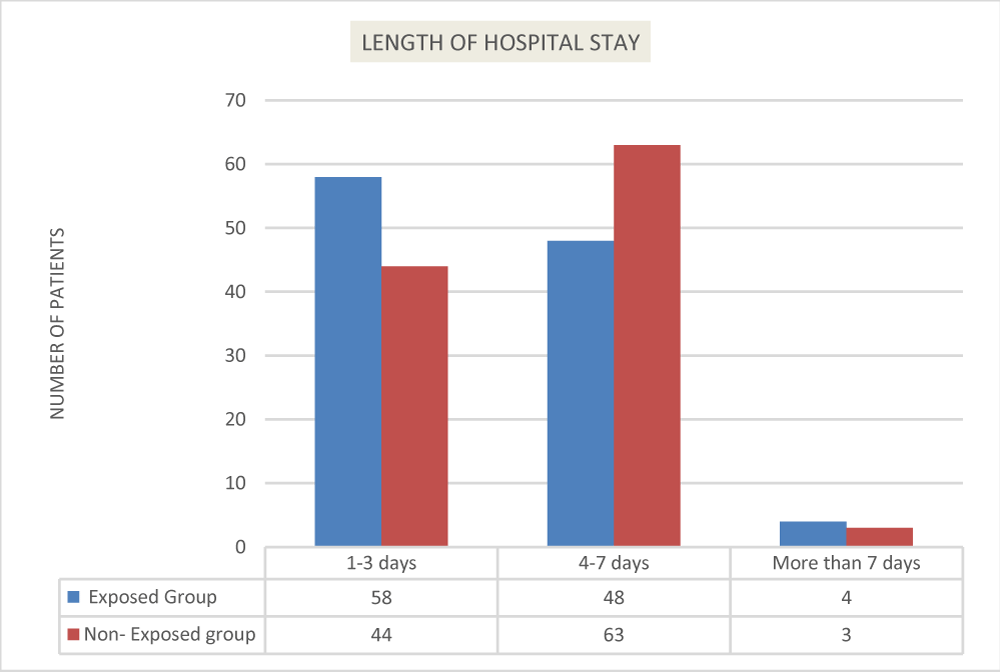
Figure 3 below shows the data bearing on the contingency between the two groups (exposed and non-exposed group) and length of hospital stay (1-3 days, 4-7 days, and more than 7 days). Examining the pattern of the frequencies, it can be noted that there is a greater frequency of pediatric patients under the non-exposure group who had a longer length of hospital stay (4-7 days and more than 7 days). On one hand, there is a greater frequency of pediatric patients under the exposure group (utilized Biofire) who had a shorter length of hospital stay (1-3 days) than pediatric patients under the non-exposure group as shown in Figure 3.
The chi-square results show that the type of group differs significantly in terms of length of hospital stay (x2 = 14.09, p = .029). The p - value (two-tailed) is smaller than the standard alpha value of 0.05, so the null hypothesis that the conditions are independent of each other should be rejected. This also implies that there is a significant association between shorter hospital stays of pediatric patients who underwent the Film Array (Biofire) respiratory panel versus pediatric patients who did not undergo the test.
Discussion
In both developed and developing countries, acute respiratory tract infection is the most common cause of hospitalization in children. Early epidemiologic studies documenting that children are at particular risk for viral respiratory infection have other findings such as a higher frequency of illnesses in females and the peak of illness is during autumn and spring season [1]. The immune status of children is different from adults, and the amount of maternal antibodies attenuate distinctly, which makes children susceptible to respiratory viral infections. This present study demonstrated that the most common age group hospitalized are ages 1-5 years old, female children, and those that resided in urban localities.
The adoption of preventive measures, including personal and home hygiene, sanitation, and sufficient ventilation, may be done throughout the peak of the ARI season if parents or other caregivers of children are aware of the seasonal variations of infections [5]. Keeping the hands clean frequently, avoiding crowded public spaces, and getting an annual flu shot are additional preventive steps. Our data demonstrated that the most children admitted are in July and October which is the rainy season in tropical countries. In addition, most patients who underwent the Film Array (Biofire) respiratory panel were tested in the latter years with the majority during July 2021, when there was an increasing number of SARS-CoV-2 cases. On the other hand, those who didn’t undergo the test were admitted in the early years when the Film Array (Biofire) was not yet familiar to most pediatricians. Moreover, the majority of SARS-CoV-2 cases were detected in August and September 2021 when there was a spike in delta variant cases in the area.
The majority in both groups were not given the influenza vaccine. This could be explained as the study population only included admitted patients. Therefore, it is expected that the unvaccinated would present a more severe course requiring admission.
Although ARIs are linked to a wide variety of infections, regardless of the underlying cause, the clinical symptoms are usually similar. Therefore, identification of the potential causal agents is a must for effective therapy, and virus detection can minimize the unnecessary and excessive use of antibiotics. The present study demonstrated that the most commonly detected viruses out of all the ARI cases are the human rhinovirus and enterovirus. Our analysis found that the infection was strongly associated with the cause of hospitalization.
Although ARIs are linked to a wide variety of infections, regardless of the underlying cause, the clinical symptoms are usually similar. Therefore, identification of the potential causal agents is a must for effective therapy, and virus detection can minimize the unnecessary and excessive use of antibiotics. The present study demonstrated that the most commonly detected viruses out of all the ARI cases are the human rhinovirus and enterovirus. At the host level, the outcome of dual infection is commonly superinfection. Co-infections can also alter the epidemiology of viral infections. It is postulated that the sequence of infections, the time interval between viral exposure, and the route of infections affect the pathogenicity of the co-infection [20]. Our data demonstrated the most common viruses with co-detection that can cause hospitalization in children are human rhinovirus and influenza B. Moreover, the profile of children with viral co-detection is majority is female, aged 1-5 years old, residing in urban localities with no history of influenza vaccination. This is most likely because the younger age group living in the more populated urban locality and without influenza vaccination are more prone to get ARI requiring admission. Our data also further demonstrated that most children with viral co-detection respiratory panel results are admitted in July and December. This follows the pattern of more viral infections during the rainy seasons.
The study showed that, compared to patients who were not evaluated using the respiratory panel, children’s medical management changed significantly when the results of a Film Array (Biofire) Respiratory panel were provided. These adjustments included a reduction in the use of antibiotics, shorter hospital stays, and rapid detection of pathogens. A study conducted by Calderaro, et al. (2022), shows that the current algorithm for the laboratory diagnosis of respiratory tract infections relies on multiple approaches including gold-standard conventional methods, among which the traditional culture is the most used, and innovative ones such as molecular methods like PCR mostly used to detect viruses and atypical bacteria. The implementation of molecular methods with syndromic panels has the potential to be a powerful decision-making tool for patient management despite requiring appropriate use of the test in different patient populations.
Traditionally, microbial culture methods serve as the foundational tests for identifying pathogens responsible for infectious diseases. It is recognized that bacterial and viral culture tests demand specialized facilities and highly trained personnel, more so than molecular biological tests. Bacterial culture typically spans from 24 hours to 5 days [21]. The confirmation process for the culture of respiratory viral pathogens, which is the primary focus of this study, can extend over several days to several weeks. In contrast, the FilmArray™ RP offers a rapid and efficient alternative, detecting respiratory pathogens within 2 h. This swift turnaround time facilitates timely decision-making regarding treatment. The expedited results provided by the FilmArray™ RP allow for a shift from empirical antibiotics, such as amoxicillin, clavulanic acid, macrolides, and doxycycline, to more targeted antibiotic prescriptions. This transition enables more precise and effective administration of antibiotics [22].
To fully comprehend the dynamics of pathogen spread and infection patterns, epidemiological research on upper respiratory tract infections requires the use of a variety of multiplex PCR techniques, such as the FilmArray RP. The importance of quick and precise diagnostic tests has become more widely acknowledged, leading to the adoption of multiplex PCR diagnostic techniques. This emphasizes how crucial cutting-edge diagnostic tools are to developing successful public health plans and initiatives.
In a previous study conducted by Rehder, et al. [23] isolation of two or more respiratory viral pathogens is associated with moderate or severe illness and death in children cared for in the hospital setting but larger prospective studies are needed to clarify patients at greatest risk and to evaluate interactions between specific viruses. Just as certain individual viruses may be more virulent, specific virus combinations may be associated with worse clinical outcomes. However, in our study, this was not associated with the severity of illness because most of the cases included in the study were all admitted in non-critical setup and discharged improved with no mortality documented.
Conclusion
Acute respiratory tract infection is a clinical diagnosis and most infections are caused by viruses. The most commonly affected age group is children ages 1-5 years old with human rhinovirus and enterovirus as the most common viral pathogens detected especially during July and October. The use of a respiratory panel to assess disease severity for the individual patient could be used for clinical decision-making in the management of children with ARIs.
The Respiratory panel test is not routinely available in many hospitals in detecting respiratory infection before the pandemic because of its cost and its restricted use in patients with a high risk of complications or with an unexpected disease course. But with the advent of the Sars-Cov-2 pandemic, the respiratory panel now is routinely used and recommended to detect the cause of severe respiratory infections in children.
In conclusion, the use of Film Array (Biofire) respiratory panels can provide clinicians with a rapid and useful diagnostic tool in the detection of respiratory viruses and atypical bacterial leading to improvement of patient care and assisting clinical judgment regarding the usage of antibiotics as well as the length of hospitalization for those with severe respiratory tract infections.
Declarations
Ethical approval and consent to participate: It was approved by the Chong Hua Hospital Institutional Review Board last November 25, 2021, with an IRB reference code IRBi-9121-10. Informed consent was obtained from all the parents and/or legal guardians for the study and it was attached to their chart during the time of their admission. All methods were carried out following relevant institutional guidelines and regulations.
Availability of data and materials: The availability of the data and material is per request from the corresponding author who is Dr. Shajed Julasiri and you can send a request for the data from this study through his email ([email protected]).
Contributors statement: Dr. Julasiri designed the project, presented the ideas and the computational framework, and gathered and analyzed the data. Dr. Julasiri with the help of Dr. Lim. carried out the implementation. Dr. Julasiri performed the calculations. Dr. Lim and Dr. Kimseng verified the analytical methods. Dr. Julasiri wrote the manuscript with input from Dr. Lim and Dr. Kimseng. All authors conceived the study and were in charge of overall direction and planning.
My department head, training officer, and research committee, who enabled me to complete this project, deserve recognition and my sincere gratitude. My reliance on their direction and counsel helped me complete this research work. I want to express my sincere gratitude to my family in particular for their unwavering assistance and patience while I was writing this research report. I’ve gotten this far thanks to your prayers for me. Finally, I want to express my gratitude to Allah for leading me and guiding me through all of the challenges I faced while writing this research paper. I will continue to put my future in your hands.
Author’s information
*Shajed A. Julasiri, MD. He is the corresponding author and senior Pediatric resident in Chong Hua Hospital Mandaue.
Jonathan G. Lim, MD, FPPS, FIDSP. He is one of the co-authors and mentors. He is a Pediatric Infectious Specialist and Assistant Chair of the Department of Pediatrics, at Chong Hua Hospital Cebu.
Karen Joy N. Kimseng MD, FPPS, is one of the co-authors. A pediatric Rheumatologist and the Research Head of Chong Hua Hospital Mandaue.
- Monto AS. Epidemiology of viral respiratory infections. Am J Med. 2002;112 Suppl 6A:4S-12S. Available from: https://doi.org/10.1016/s0002-9343(01)01058-0
- Reischl AT, Schreiner D, Poplawska K, Kidszun A, Zepp F, Gröndahl B, et al. The clinical impact of PCR-based point-of-care diagnostic in respiratory tract infections in children. J Clin Lab Anal. 2020;34(5):e23203. Available from: https://doi.org/10.1002/jcla.23203
- Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302(7):758-66. Available from: https://doi.org/10.1001/jama.2009.1163
- Schulert GS, Lu Z, Wingo T, Tang YW, Saville BR, Hain PD. Role of a respiratory viral panel in the clinical management of pediatric inpatients. Pediatr Infect Dis J. 2013;32(5):467-72. Available from: https://doi.org/10.1097/inf.0b013e318284b146
- Giamberardin HI, Homsani S, Bricks LF, Pacheco AP, Guedes M, Debur MC, et al. Clinical and epidemiological features of respiratory virus infections in preschool children over two consecutive influenza seasons in southern Brazil. J Med Virol. 2016;88(8):1325-33. Available from: https://doi.org/10.1002/jmv.24477
- Litwin CM, Bosley JG. Seasonality and prevalence of respiratory pathogens detected by multiplex PCR at a tertiary care medical center. Arch Virol. 2014;159(1):65-72. Available from: https://doi.org/10.1007/s00705-013-1794-4
- Leber AL, Everhart K, Daly JA, Hopper A, Harrington A, Schreckenberger P, et al. Multicenter Evaluation of BioFire FilmArray Respiratory Panel 2 for Detection of Viruses and Bacteria in Nasopharyngeal Swab Samples. J Clin Microbiol. 2018;56(6):e01945-17. Available from: https://doi.org/10.1128/jcm.01945-17
- Biofire® respiratory 2.1 plus panel. (2021, February 22). bioMérieux Clinical Diagnostics. Accessed 10 Feb 2021. Available from: https://www.biomerieux.com/corp/en/our-offer/clinical-products/biofire-respiratory-2-1-panels.html
- Rogers BB, Shankar P, Jerris RC, Kotzbauer D, Anderson EJ, Watson JR, et al. Impact of a rapid respiratory panel test on patient outcomes. Arch Pathol Lab Med. 2015;139(5):636-41. Available from: https://doi.org/10.5858/arpa.2014-0257-oa
- Andrews D, Chetty Y, Cooper BS, Virk M, Glass SK, Letters A, et al. Multiplex PCR point of care testing versus routine, laboratory-based testing in the treatment of adults with respiratory tract infections: a quasi-randomised study assessing impact on length of stay and antimicrobial use. BMC Infect Dis. 2017;17(1):671. Available from: https://doi.org/10.1186/s12879-017-2784-z
- Lee BR, Hassan F, Jackson MA, Selvarangan R. Impact of multiplex molecular assay turn-around-time on antibiotic utilization and clinical management of hospitalized children with acute respiratory tract infections. J Clin Virol. 2019;110:11-16. Available from: https://doi.org/10.1016/j.jcv.2018.11.006
- Barenfanger J, Drake C, Leon N, Mueller T, Troutt T. Clinical and financial benefits of rapid detection of respiratory viruses: an outcomes study. J Clin Microbiol. 2000;38(8):2824-8. Available from: https://doi.org/10.1128/jcm.38.8.2824-2828.2000
- Wishaupt JO, Versteegh FG, Hartwig NG. PCR testing for paediatric acute respiratory tract infections. Paediatr Respir Rev. 2015;16(1):43-48. Available from: https://doi.org/10.1016/j.prrv.2014.07.002
- Echavarría M, Marcone DN, Querci M, Seoane A, Ypas M, Videla C, et al. Clinical impact of rapid molecular detection of respiratory pathogens in patients with acute respiratory infection. J Clin Virol. 2018;108:90-95. Available from: https://doi.org/10.1016/j.jcv.2018.09.009
- Linder JA. Editorial commentary: antibiotics for treatment of acute respiratory tract infections: decreasing benefit, increasing risk, and the irrelevance of antimicrobial resistance. Clin Infect Dis. 2008;47(6):744-6. Available from: https://doi.org/10.1086/591149
- Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128(6):1053-61. Available from: https://doi.org/10.1542/peds.2011-1337
- Wishaupt JO, Russcher A, Smeets LC, Versteegh FG, Hartwig NG. Clinical impact of RT-PCR for pediatric acute respiratory infections: a controlled clinical trial. Pediatrics. 2011;128(5):e1113-20. Available from: https://doi.org/10.1542/peds.2010-2779
- Subramony A, Zachariah P, Krones A, Whittier S, Saiman L. Impact of Multiplex Polymerase Chain Reaction Testing for Respiratory Pathogens on Healthcare Resource Utilization for Pediatric Inpatients. J Pediatr. 2016;173:196-201.e2. Available from: https://doi.org/10.1016/j.jpeds.2016.02.050
- Brendish NJ, Malachira AK, Armstrong L, Houghton R, Aitken S, Nyimbili E, Ewings S, Lillie PJ, Clark TW. Routine molecular point-of-care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open-label, randomised controlled trial. Lancet Respir Med. 2017 May;5(5):401-411. Available from: https://doi.org/10.1016/s2213-2600(17)30120-0
- Meskill SD, O'Bryant SC. Respiratory Virus Co-infection in Acute Respiratory Infections in Children. Curr Infect Dis Rep. 2020;22(1):3. Available from: https://doi.org/10.1007/s11908-020-0711-8
- Lagier JC, Edouard S, Pagnier I, Mediannikov O, Drancourt M, Raoult D. Current and past strategies for bacterial culture in clinical microbiology. Clin Microbiol Rev. 2015;28(1):208-36. Available from: https://doi.org/10.1128/cmr.00110-14
- Panasiuk L, Lukas W, Paprzycki P, Verheij T, Godycki-Ćwirko M, Chlabicz S. Antibiotics in the treatment of upper respiratory tract infections in Poland. Is there any improvement? J Clin Pharm Ther. 2010;35(6):665-9. Available from: https://doi.org/10.1111/j.1365-2710.2009.01136.x
- Rehder KJ, Wilson EA, Zimmerman KO, Cunningham CK, Turner DA. Detection of Multiple Respiratory Viruses Associated With Mortality and Severity of Illness in Children. Pediatr Crit Care Med. 2015;16(7):e201-6. Available from: https://doi.org/10.1097/pcc.0000000000000492
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
