Archives of Pulmonology and Respiratory Care
Coronary Artery Bypass Surgery in Left Main Disease with Idiopathic Artery Pulmonary Hypertension. Role of PGE Therapy
Walter Serra1*, Igino Spaggiari2, Alessandro Maria Budillon2, Filippo Benassi2, Giorgio Romano2, Tiziano Gherli2, Antonella Vezzani2 and Tullio Manca2
2Heart Surgery Institute, Surgery Department, University Hospital Parma, Italy
Cite this as
Serra W, Spaggiari I, Budillon AM, Benassi F, Romano G, et al. (2016) Coronary Artery Bypass Surgery in Left Main Disease with Idiopathic Artery Pulmonary Hypertension. Role of PGE Therapy. Arch Pulmonol Respir Care 2(1): 024-027. DOI: 10.17352/aprc.000012Introduction
Idiopathic pulmonary hypertension (PAH) is a rare disease of unknown etiology that leads to the development of severe precapillary pulmonary hypertension [1-3], characterized by impaired regulation of both pulmonary hemodynamics and vascular growth.’ The responsiveness to vasodilator therapy in patients with PAH varies considerably [4,5]. Coronary artery bypass grafting (CABG) with extracorporeal circulation has a deleterious effect on lung tissues.
Left coronary artery could be compressed by the enlargement of the main pulmonary artery.
Postoperative pulmonary hypertension contributes to increased perioperative mortality and in patients undergoing CABG, was the only independent variable able to predict postoperative mortality. To date, right ventricular dysfunction, as surgical risk score, is not considered.
The main pathophysiologic mechanism of this dysfunction is the afterload mismatch.
The right ventricular (RV) contractility is increased, but not enough compared to the afterload.
Case Report
We present the case of a patient of 78 years with PAH with a medical history of blood hypertension and former smoking status. Tromboendoarterectomy (TEA) of left carotid. Prostatectomy for adenocarcinoma. Chronic kidney failure in nephrology follow-up for about two years (serum creatinine home to around 1.3 mg / dl).
From about two years he was followed for pulmonary hypertension in patients symptomatic for exertional dyspnea (NYHA class II). Performed chest Computed Tomographic (CT) scan that showed no pulmonary thromboembolism (TEP). Pulmonary Artery diameter: 35 mm. Aorta with atheromatous plaques. Multiple mixed-type ulcerated, mainly to ‘bow and downslope. Presence of isolated bubbles of emphysema in the apical and basal right.
6 Minutes Walking Test (6MWT) showed a reducing the distance traveled 286 meter. Basal O2 Sat 98%. Mean Sat O2 91%. Was recorded oxyhemoglobin desaturation is not clinically relevant.
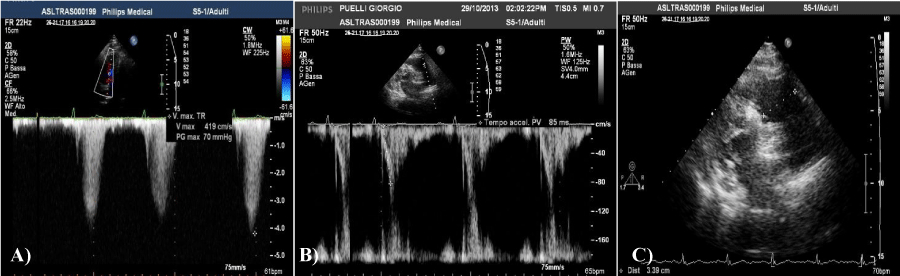
Spirometry: normal volumes, flows, and resistances. Moderate-to-severe reduction in the transfer factor of CO: (DLCO 40%). Transthoracic Echocardiogram (TTE): Left ventricular ejection fraction (FEVsx) preserved (57%); pulmonary hypertension estimated severe (grad AD / VD 70 mmHg, 75 mmHg of systolic pulmonary artery pressure (PAPs). Tricuspid annular systolic excursion (TAPSE) was 18; Acceleration time of pulmonary outflow (Actpo) 85; mild concentric hypertrophy. Pulmonary artery trunk dilatation Figure 1,a,b,c.a
It was given an indication for catheterization right assessment in anticipation of possible start of specific drug therapy. Right-heart catheterization and vasoreactivity study and coronary angiography showed:
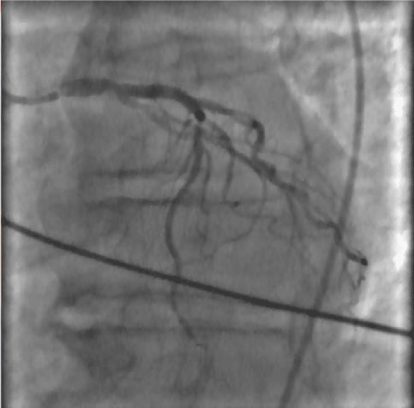
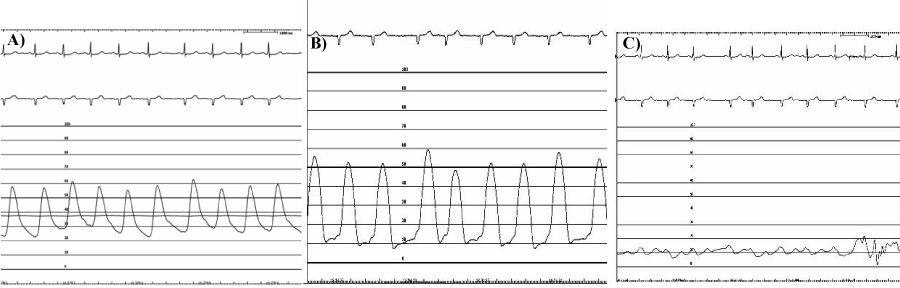
Left main coronaric trunk with critical stenosis of 60% pre-divisional Figure 2, anterior descending artery with well-developed critical stenosis (80%) in the middle part; circumflex branch extensively in the absence of atheromatous stenosis criticism; dominant right coronary atheromatous extensively with critical stenosis to distal segment; Left ventricular volumes at the upper limits with marked hypertrophy parietal and global kinetic moderately depressed (FE 45%), the absence of significant mitral regurgitation; aortography with aortic cusps furniture devoid of significant regurgitation. Severe Pulmonary Precapillary Hypertension was detect : mean pulmonary artery pressure ( mPAP) 40 mm/Hg, postcapillary wedge pressure (PCWP) 10mm/Hg, pulmonary vascular resistance (PVR) 6 UW, cardiac index (IC) 1.5 L/min/m2, not vasoreactive Figure 3a,b,c.
Among other investigations carried out: Eco TSA: left widespread intima-media thickening and slight atheroma of the bulb in outcomes of TEA. Right diffuse intima-media thickening and plaque predominantly hypoechoic in charge of the internal carotid artery proximal determinant stenosis of 30-40%.
The main blood tests: white blood cell count normal, Hb 10.7 g / dl, creatinin 1.5 mg/dl, K 4.2 mEq/L. Platelet 138.000.
Secondary pulmonary hypertension from heart and pulmonary disease, sleep associated disorders, chronic thromboembolic disease, autoimmune or collagen vascular disease, HIV infection, or liver disease were ruled out.
The patient was diagnosed with PAH and coronary artery disease.
In light of the data was performed angiographic consulting Cardiac Surgery that posed indication for surgical myocardial revascularization with high operative risk.
In cardiac surgery patient seemed sufficiently compensated with Euroscore 9.
The patient underwent to coronary artery bypass graft (CABG) surgery with cardiopulmonary Bypass. After longitudinal median sternotomy was isolated the left internal mammary artery and at same time, was isolated and harvested the left Great Saphenous Vein (SVG). Then, after pericardiotomy and exposure of the heart and the great vessels, was started CPB placing cannulas in Ascending Aorta and in Inferior Vena Cava. The heart was arrested by cardioplegic solution infusion slightly hypotermic in aortic bulb and in coronary sinus.
Postoperatively it showed a low cardiac index requiring inotropic support: dopamine 400 mg; 250 mg dobutamine, norepinephrine 10mg.
Nevertheless the hemodynamic response was very poor (IC 1.7) for which we have associated with the therapy alprostadil (PGE1) intravenously at the starting dose of 2.5ng / kg / min until the maximum tolerated dose of 20 ng / kg / min in continuous infusion pump for three consecutive days.
Outcome
Echocardiographic data : PAPs from 70mm/Hg to 63mm/Hg; TAPSE from 12 mm to 16mm; Actpo from 85 to 90 msc Haemodinamic data : mPAP from 42mm/Hg to 35mm/Hg, PVR from 6 UW to 3.8 UW, IC from 1.5 to 2 L/min/m2 . PaO2 61 to 78 and SaO from 95% to 99%.
At three months follow-up patient was in NYHA Class II .An echocardiographic exam showed these data: PAPs 75mm/Hg, TAPSE 18;Actpo 88.
We started Sildenafil 20mg/3/die with improvement of 65 meter at six minutes walking test (6MWT) performed after three months.
Discussion
PAH is found predominantly in young adult females and mean age of the distribution is 35years.
Angina is most likely related to right ventricular ischemia secondary to high myocardial oxygen demand provoked by increased ventricular wall tension and increased right ventricular mass. PAH and coronary artery disease are extremely rare clinical conditions. Prostaglandin E1 (PGE1) is a potent vasodilating drug, which has been used in the treatment of primary pulmonary hypertension to reduce pulmonary vascular resistances (PVR).
An effect on the systemic resistances occurs only for infusion doses greater than 20ng / kg / min 6.
Documented neuro-hormonal effects have been reported by Hulsmann, through a reduction of atrial natriuretic factor and norepinephrine on the one hand and increased renal blood flow, with increased diuresis [6-10].
This action occurs through an antagonistic effect of PGE1 on Angiotensin II at the level of systemic vascular resistance, on the excretion of sodium and water, and through a mechanism that reduces apoptosis in ischemic hearts.
It has been demonstrated a modulating effect on the production of cytokines during coronary angioplasty and bypass during coronary artery [11,12].
However intravenous PGE1 infusion may be of benefit and also has been proposed as a therapeutic tool in patients with end-stage heart failure and after residual pulmonary hypertension following pulmonary endarterectomy [7,8,13,16,]./p>
The pathophysiological rationale for the use of prostaglandins in heart failure is based on the results published in the literature have documented hemodynamic effects with reduction in pulmonary vascular resistances and increase in cardiac index, through a mechanism in the absence of endothelium-dependent increase on heart rate [4,5,14,15,17].
Right ventricular failure due to persistent elevation of pulmonary pressure is the most important complication after CABG. Recurrent severe bradycardia and cardiac arrest can develop easily due to acute right ventricular failure. The main pathophysiologic mechanism result from worsened longitudinal shortening and the septal oblique fiber orientation damage [18]. The right ventricular contractility is preserved by transverse basal fiber if PVR is low.
An heroic therapy in the postoperative period is right ventricular decompression by creating an atrial septostomy, but the most important problem is the right ventricular failure.
PGE continuous infusion is an alternative therapeutic modality after CABG surgery in patients with PAH. PAH-related hypoxia and after-load mismatch can be overcome by using vasodilator therapy (PGE1) in the post-pump period.
Conclusions
Pulmonary hypertension and its most consequence, RV dysfunction, are important mortality risk factors in cardiac surgery. In the case we report which therapy with prostaglandin (alprostadil) has been effective in allowing weaning post-operative conditions of right ventricular failure with pulmonary hypertension.
Accordingly, all cardiac patients may benefit from early diagnosis and/or treatment prior to the surgical procedure.
Thus, future trials should prioritize in-depth exploration of preventive approaches as prostaglandin infusion, in order to address the role of preemptive reduction of PH severity before cardiac surgery and to determine its impact on postoperative outcomes and survival improvement.
WS: Performed the study and wrote the paper; IS,AMB, FB,GR, VA,TM,WS. They performed the surgery and postoperative therapy TG approves the study.
- Wagenvoort CA, Wagenvoort N (1970) Primary pulmonary hypertension: A pathological study of the lung vessels in 156 clinically diagnosed cases. Circulation 42: 1163.
- Weir EK, Rubin LJ, Ayres SM (1989) The acute administration of vasodilator therapy in primary pulmonary hypertension.Experience from the National Institutes of Health Registry on primary pulmonary hypertension. Am Rev Respir Dis 140: 1623-1630.
- Rich S, Dantzker DR, Ayres SM (1987) Primary pulmonary hypertension: A national prospective study. Ann Intern Med 107: 216-223.
- Olsson AG, Carlson LA (1976) Clinical, hemodynamic and metabolic effects of intra-arterial infusions of prostaglandin E1 in patients with peripheral vascular disease. Adv Prostaglandin Thromboxane Res 1: 429-432.
- Murali S, Kormos RL, Uretsky BF, Schechter D, Reddy PS, et al. (1993) Preoperative pulmonary hemodynamics and early mortality after orthotopic cardiac transplantation: the Pittsburgh experience. Am Heart J 126: 896-904.
- Wutte M, Hülsmann M, Berger R, Rödler S, Frey B, et al. (1998) Improved kidney function with intravenous prostaglandin E1 in patients with terminal heart failure. Wien Klin Wochenschr 110: 473-478.
- Pacher R, Stanek B, Hülsmann M, Sinzinger H (1999) Effects of prostaglandin E1, dobutamine and placebo on hemodynamic, renal and neurohormonal variables in patients with advanced heart failure. Jpn Heart J 40: 321-335.
- Pacher R, Stanek B, Hülsmann M, Berger R, Siegel A, et al. (1997) Prostaglandin E1-bridge to cardiac transplantation. Technique, dosage, results. Eur Heart J 18: 318-329.
- Taylor MB, Laussen PC (2010) Fundamentals of management of acute postoperative pulmonary hypertension. Pediatric Crit Care Med 11: S27–29.
- Fraisse A, Butrous G, Taylor MB, Oakes M, Dilleen M, et al. (2011) Intravenous sildenafil for postoperative pulmonary hypertension in children with congenital heart disease. Intensive Care Med 37: 502–509.
- Galie N, Hoeper MM, Humbert M, et al. (2009) Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 30: 2493–2537.
- Dong MF, Ma ZS, Ma SJ, Chai SD, Tang PZ, et al. (2012) Effect of prostaglandin E1 on pulmonary arterial hypertension following corrective surgery for congenital heart disease. J Cardiovasc Pharmacol Ther 17: 303–307.
- Kramm T, Eberle B, Guth S, Mayer E (2005) Inhaled iloprost to control residual pulmonary hypertension following pulmonary endarterectomy. Eur J Cardiothorac Surg 28: 882–888.
- D’Ambra MN, LaRaia PJ, Philbin DM, Watkins WD, Hilgenberg AD, et al. (1985) Prostaglandin E1. A new therapy for refractory right heart failure and pulmonary hypertension after mitral valve replacement. J Thorac Cardiovasc Surg 89: 567–572.
- Kermode J, Butt W, Shann F (1991) Comparison between prostaglandin E1 and epoprostenol (prostacyclin) in infants after heart surgery. Br Heart J 66: 175–178.
- Rubis LJ, Stephenson LW, Johnston MR, Nagaraj S, Edmunds LH Jr (1981) Comparison of effects of prostaglandin E1 and nitroprusside on pulmonary vascular resistance in children after open-heart surgery. Ann Thorac Surg 32: 563–570.
- Serra W, Musiari L, Ardissino D, Gherli T, Montanari A (2011) Benefit of prostaglandin infusion in severe heart failure: Preliminary clinical experience of repetitive administration. Int J Cardiol 146: e10–15.
- Ngujen T, Cao L (2014) Alterated Right Ventricle Contractile Pattern after Cardiac Surgery: Monitoring of Septal Function is Essential. Echocardiography 31: 1159-1165.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
