Archives of Pulmonology and Respiratory Care
Oxidative Stress, Antioxidant Status and Inflammation in Chronic Bronchitis and Pulmonary Emphysema
1Pulmonary Department of the Hospitalar Center of Occidental Lisbon-Egas Moniz Hospital, Lisbon, Portugal
2Lusophone University of Humanities and Technology of Lisbon
3Laboratory of Genetics, Faculty of Medicine, University of Lisbon, Portugal
Author and article information
Cite this as
Cristóvão C, Cristóvão L, Nogueira F, Bicho M (2017) Oxidative Stress, Antioxidant Status and Inflammation in Chronic Bronchitis and Pulmonary Emphysema. Arch Pulmonol Respir Care. 2017; 3(1): 001-006. Available from: 10.17352/aprc.000015
Copyright License
© 2017 Cristóvão C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: Chronic Obstructive Pulmonary Disease (COPD) is characterized by a complex range of pathological changes including both pulmonary and systemic effects. Several mechanisms contribute to the variable intermediate and clinically relevant disease phenotypes, such as chronic bronchitis and emphysema, and systemic disease. The molecular mechanisms associated to the pathogenesis of COPD are not yet clearly understood.
Objective: The aim of this study was to evaluate oxidant/antioxidant balance and the systemic inflammation in chronic bronchitis and pulmonary emphysema COPD patients.
Methods: We analyzed COPD patients divided in 2 groups: chronic bronchitis and emphysema. Healthy volunteers without lung disease were used as control group.
Results: We observed a significant (P<0,05) increase in the levels of plasma malondialdehyde (MDA), a marker of oxidative stress, an increase in the circulating C-reactive protein (CRP), used as a biomarker of systemic inflammation, and a decrease in antioxidant defense in COPD patients with emphysema when compared with COPD patients with chronic bronchitis.
Conclusion: Althought our results should be regarded as preliminary they indicate a disturbance in oxidant/antioxidant status and systemic inflammatory response associated to COPD patients. The differences observed were more evident in emphysematous phenotype.
Chronic obstructive pulmonary disease (COPD) is characterized by an airflow limitation that is not fully reversible and is usually progressive and associated with an abnormal inflammatory response to noxious particles and gases [1,2]. Although COPD affects the lungs, it also produces significant systemic consequences [2]. The systemic effects associated to COPD are clinically relevant and may contribute to better understanding and management of the disease [3,4]. Several studies demonstrated that an oxidant/antioxidant imbalance play an important role in the pathogenesis of COPD [5-10]. The oxidative stress in patients with COPD derives from the increased of oxidants present in cigarette smoke and/or from the increased amounts of reactive oxygen species released from leucocytes, both in air spaces and in blood [8]. Although there is strong evidence of oxidative stress in the pathogenesis of COPD, the mechanisms associated to the heterogeneity of the disease are not understood. The typical clinical manifestations of the COPD syndrome include chronic bronchitis, a condition of large-airway inflammation and remodeling, and emphysema, disease of the distal airways and lung parenchyma that manifests as loss of surface area for gas exchange [11]. The majority of the information associated to the pathogenesis of COPD is related to the emphysema. It has been reported that the pathogenesis of COPD, particularly of emphysema, which involves the destruction of small airway structures and alveolar units, is potentiated by interactions between apoptosis, oxidative stress, and protease/antiprotease imbalance [12-14]. It has been suggested that the factors that initiate inflammation and the effects of proteolytic and oxidant-induced damage may be also important in the other COPD conditions, namely chronic bronchitis [6]. There is growing evidence that new markers are needed to provide further insights into the pathogenesis of COPD [15].
The aim of this study was to evaluate oxidant/antioxidant balance and systemic inflammation in chronic bronchitis and pulmonary emphysema.
Methods
Study subjects
The present study assessed the involvement of oxidants/antioxidants balance and systemic inflammation in two groups of subjects with clinically stable COPD and with different phenotypes- chronic bronchitis and emphysema.
Fifty healthy subjects (mean age 41,60±12,31 years; 12 female, 38 male and body mass index, BMI 27,322±4,310 kg/m2) having no history of respiratory or atopic disease and free of any medication were used as control group. Twelve of them were current smokers and thirty eight had never smoked.
Patients with stable COPD were study. COPD was defined according to the criteria of the Global Initiative for Chronic Obstructive Lung Disease (GOLD). Lung function was assessed with body plethysmography. COPD patients were recruited from the respiratory outpatient consultation. Data from medical history were collected, with focus in smoking history, respiratory simptoms and signs. Lung function were evaluated for diagnosis and classification of severity of COPD, according to the GOLD criteria. Carbon monoxide gas diffusion were also performed. The characteristics of COPD group are shown in Table 1. The patients were grouped in chronic bronchitis and emphysema. The patients were included in Emphysema group if they had emphysema by high resolution computed tomography and dyspnea on exertion. Were exclusion criteria the presence of others respiratory diseases or malignancy and acute exacerbation. The COPD patients were grouped as follows: patients with chronic bronchitis (n=11) and patients with emphysema (n=9). The clinical and physiological characteristics of COPD phenotypes are shown in Table 2.
The study was conducted according to the rules of the declaration of Helsinki. The ethical conduct was ensure in this investigation and informed written consent was obtained from all subjects participating in the study.
Oxidative stress
One of the main targets of oxidative stress are the polyunsaturated fatty acids present in cell membranes, leading to lipid peroxidation [16,17]. Malonyldialdehyde (MDA) is a product resulting from lipid peroxidation. The evaluation of MDA in biological samples is considered an indicator of increased lipid peroxidation and is used as an oxidative stress biomarker [16,18,19]. MDA concentrations were measured spectrophotometrically in terms of thiobarbituric acid reactive substances (TBARS) using a spectrophotometric method modified from Okhawa et al. [16]. The absorbances were read at 532 nm corresponding to the colored complex formed between the MDA and thiobarbituric acid (TBA). The concentration of TBARS was calculated using the MDA concentration and using a calibration curve previously prepared. The concentration of MDA was expressed in nmol/mL of plasma.
Antioxidant plasma status
Antioxidant plasma status was evaluated by the quantification of vitamin C and total sulphydryl groups. Vitamin C was determined based on the method described by Omaye et al. [21]. In brief, the dinitrophenylhydrazine reacts with oxidized vitamin C (oxidized ascorbic acid) to give a colored product. The absorbance was measured at 520 nm and is directly proportional to the vitamin C concentration. The levels of vitamin C were expressed in µg/mL of plasma.
The quantifications of total sulphydryl groups were performed according to the method described by Rice-Evans et al. [22]. The non-protein sulphydryl groups are mainly in the form of reduced glutathione (GSH). We evaluated the sulphydryl group using a spectrophotometric method involving the use of Ellman´s Reagent. The 5,5’- Dithiobis(2-nitrobenzoic acid) (DTNB) undergoes disulfide exchange with sulphydryl groups and occurs the formation of 5-thio-2-nitrobenzoate anion (TNB). The absorbance of the reduced chromogen was measured at 412 nm and is directly proportional to the GSH concentration. The levels of GSH were expressed in µmol/mL of plasma.
Inflammation
Circulating inflammatory mediator was assessed by the measurement of the acute phase protein C-reactive protein (CRP) levels, a marker of systemic inflammation. CRP levels were measured by chemioluminescent immunoassay. The levels of CRP were expressed in mg/L.
Statistical analysis
The results are expressed as mean ± SEM of the concentrations of plasma parameters evaluated. The appropriate nonparametric test was chosen for data not normally distributed. Comparisons between two groups were tested using unpaired t-test or Mann-Whitney U test. A difference with P<0,05 was considered statistically significant.
Results
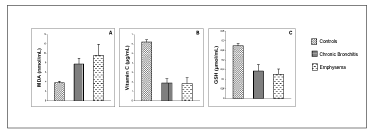
The plasma concentrations of MDA, vitamin C and GSH of COPD patients with chronic bronchitis and COPD patients with emphysema are shown in Table 3.
The plasma MDA levels found to be significantly (P<0,001) higher in COPD patients with chronic bronchitis and with emphysema when compared with control group (3,830±0,310 nmol/mL). Additionally, our results have shown a significant (P<0,05) increase in the marker of oxidative stress associated to emphysematous COPD patients when compared to bronchitis Figure 1.
The two sub-groups of COPD patients had a significant (P<0,001) decrease in antioxidant status in chronic bronchitis and emphysema when compared with control group (vitamin C 6,230±0,220 µg/mL; GSH 0,275±0,011 µmol/mL). However, no significant difference was observed when compared chronic bronchitis and emphysema. But the emphysematous patients have a decrease in the antioxidant status Figure 1.
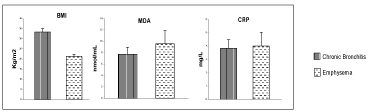
In our study, we observed a significant (P< 0,001) decrease in Body Mass index (BMI) and a significant (p<0,05) increase in plasma MDA levels in COPD patients with predominant emphysema when compared with COPD patients with predominant chronic bronchitis Figure 2.
There was no significant difference in CRP levels between COPD patients with emphysema and chronic bronchitis. But we observed a tendency toward an increase associated to emphysematous patients Figure 2.
Although our results should be regarded as preliminary, we observed that low BMI (<21 kg/m2) could be related with higher levels of CRP and higher MDA levels in COPD patients with emphysema. In addition, our results indicated that this increase in oxidative biomarker and systemic inflammation is more evident in group of emphysema with low BMI and with very severe COPD.
Discussion
We observed differences in plasmatic markers in the 2 typical clinical phenotypes studied. There was an increase in MDA and CRP levels and a decrease in antioxidant status (vitamin C and GSH) associated to emphysematous patients when compared with chronic bronchitis. Additionally, an increase in MDA and CRP levels were associated to patients with emphysematous phenotype with low BMI.
Our results agree with recent studies that have shown that COPD is associated not only with an abnormal inflammatory response of the lung parenchyma but also with a systemic inflammation and a systemic oxidative stress [3]. There is evidence of an increase in circulating CRP levels associated to stable COPD patients and this acute-phase protein has been regarded as a valid biomarker of systemic inflammation in these patients [23-26]. Some authors investigate the relationship between systemic inflammation, oxidative stress, body mass index and lung function. Karadag et al. [23], reported that CRP levels are higher in COPD patients with low BMI. Other authors suggest that low BMI is associated with higher degrees of bronchial obstruction and pulmonary hyperinflation, in association to circulating CRP levels during acute exacerbations of COPD [27]. However, other studies found increases in CRP levels with increasing BMI in stable patients with moderate to severe COPD [28]. In another study there was no correlation between BMI and MDA in patients with COPD [29]. Most studies of markers of inflammation and oxidative stress in COPD have analyzed the results for all types of patients together and the results are inconclusive. In our study, we observed a significant (P< 0,001) decrease in Body Mass index (BMI) and a significant (P<0,05) increase in plasma MDA levels in COPD patients with predominant emphysema when compared with COPD patients with predominant chronic bronchitis. Although our results should be regarded as preliminary, we observed that low BMI (<21 kg/m2) could be related with higher levels of CRP and higher MDA levels in more severe COPD patients with emphysema. Some recent studies demonstrate differences in pulmonary and systemic markers associated to emphysema and chronic bronchitis. In contrast with our results, other authors suggest that in COPD patients with emphysematous phenotype the inflammatory response and oxidative stress were reduced when compared with chronic bronchitis [30]. More studies are needed to clarify the mechanisms involved in inflammatory response and oxidant/antioxidant balance in different clinical phenotypes of COPD patients. In this study, we only evaluate systemic markers and we don’t use EBC samples. Another limitation of our study was the classification of patients by phenotype. Indeed, no well-established cutoffs are currently available. We use the computed tomography images of the thorax and the clinical characteristics to confirm the classification of patients by phenotypes. Additionally, our results should be regarded as preliminary because of the sample size. But the consistency of our results, showing differences in systemic markers between the 2 COPD phenotypes suggests that different clinical phenotypes have different systemic responses and many mechanisms may be involved in the pathogenesis of COPD. Our results, could contribute to consolidate the association between oxidative stress and COPD phenotypes. Most of the recent research has been directed to validated biomarkers to a more complete and clinically characterization of COPD [24,31-33]. The concept of an oxidant/antioxidant imbalance (oxidative stress) in the lung tissue of patients with COPD may be at the center of the pathobiology of these diseases. Oxidative stress may be one mechanism that enhances the inflammatory response [34]. It has been proposed that inflammation is related to oxidative stress, protease-antiprotease imbalance and apoptosis as destructive processes in emphysema [14,34]. In this study, we observed a statistically (P<0,05) significant increase in the biomarker of oxidative stress in patients with emphysema when compared with patients with chronic bronchitis. We didn´t find a difference in the antioxidants status (vitamin C and GSH) between the 2 phenotypes but we observe a tendency toward a decrease in antioxidant status associated to COPD patients with mainly emphysema. Other authors found a significant increase in MDA levels and a decrease in vitamin C associated COPD patients and in emphysema but an increase in GSH levels was observed in patients with emphysema [35]. There are a limited number of studies about oxidants/antioxidants in COPD phenotypes. Strong evidence for the role of oxidative stress in the protease/antiprotease imbalance and apoptosis associated to the pathogenesis of emphysema has been provided by studies in animal models of emphysema [13,36]. Other authors reported an association of emphysema with genetic variations in antioxidant genes and a decrease in the antioxidant capacity [37]. Our results show an association between COPD and insufficiency antioxidant capacity to prevent oxidative damage. More evidence of a disturbance in oxidant/antioxidant status was found in pulmonary emphysema. In addition, our results suggested that an increase in oxidative biomarker and systemic inflammation is more evident in the group of emphysema with low BMI and with very severe COPD. However, since our study population was limited size, these findings should be confirmed in further studies.
Conclusions
Althought our results should be regarded as preliminary they indicate a disturbance in oxidant/antioxidant status and systemic inflammatory response associated to COPD patients. The differences observed were more evident in emphysematous phenotype. These results could lead to better understanding of the variability associated to COPD patients and may contribute to the development of novel therapeutic interventions. Further studies are needed to analyze pathophysiological mechanisms involved in the phenotypes of COPD associated to oxidant/antioxidant imbalance.
- Barnes PJ, Shapiro SD, Pauwels RA (2003) Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 22: 672-688. Link: https://goo.gl/6ES7l5
- Celli BR, MacNee W, Agusti A, Anzueto A, Berg B, et al. (2004) Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 23: 932-946. Link: https://goo.gl/ZZ0XsA
- Agusti AGN, Noguera A, Sauleda J, Sala E, Pons J, et al. (2003) Systemic effects of chronic obstructive pulmonary disease. Eur Respir J 21: 347-360. Link: https://goo.gl/D06Xj1
- Anderson D, MacNee W (2009) Targeted treatment in COPD: a multi-system approach for a multi-system disease. Int J Chron Obstruct Pulmon Dis 4: 321-335. Link: https://goo.gl/ZQEwqO
- Repine JE, Bast A, Lankhorst I (1997) Oxidative stress in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 156: 341-357. Link: https://goo.gl/79zSVL
- MacNee W (2000) Oxidants/Antioxidants and COPD. CHEST 117: 303S-317S. Link: https://goo.gl/ssPnS4
- MacNee W (2005) Pulmonary and systemic oxidant/antioxidant imbalance in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2: 50-60. Link: https://goo.gl/UlPYdt
- Rahman I, Adcock IM (2006) Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J 28: 219-242. Link: https://goo.gl/qpmLsw
- Hanta I, Kocabas A, Canacankatan N, Kuleci S, Seydaoglu G (2006) Oxidant-Antioxidant balance in Patients with COPD. Lung 184: 51-55. Link: https://goo.gl/GqCYvH
- Cristóvão C, Cristóvão L, Nogueira F (2013) Evaluation of the oxidant and antioxidant balance in the pathogenesis of chronic obstructive pulmonary disease. Rev Port Pneumol 19: 70-75. Link: https://goo.gl/7dTOhR
- Tuder RM, Petrache I (2012) Pathogenesis of chronic obstructive pulmonary disease. J Clin Invest 122: 2749-2755. Link: https://goo.gl/zv0CLn
- Demedts IK, Demoor T, Bracke KR, Guy F Joos and Guy G Brusselle (2006) Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res 7: 3-10. Link: https://goo.gl/k8qOoP
- Tuder RM, Petrache I, Elias JA, Voelkel NF, Henson PM (2003) Apoptosis and emphysema the missing link. Am J Respir Cell Mol Biol 28: 551-554. Link: https://goo.gl/lTpyCz
- Tuder RM, Janciauskiene SM, Petrache I (2010) Lung disease associated with α1-antitrypsin deficiency. Proc Am Thorac Soc 7: 381-386. Link: https://goo.gl/aTzOGR
- Barker BL, Brightling CE (2013) Phenotyping the heterogeneity of chronic obstructive pulmonary disease. Clin Sci 124: 371-387. Link: https://goo.gl/VOUkAT
- Gutteridge JMC (1995) Lipid Peroxidation and Antioxidants as Biomarkers of Tissue Damage. Clin Chem 41: 1819-1828. Link: https://goo.gl/kWAWKl
- Bartoli ML, Novelli F, Costa F, Malagrinò L, Melosini L, et al. (2011) Malondialdehyde in Exhaled Breath Condensate as a Marker of Oxidative Stress in Different Pulmonary Diseases. Mediators of Inflammation 1-6. Link: https://goo.gl/5pctrM
- Yanbaeva DG, Dentener MA, Creutzberg EC, Wesselin G, Wouters EFM, (2007) Systemic Effects of Smoking. CHEST 131: 1557-1566. Link: https://goo.gl/E11Lkl
- Veskoukis AS, Nikolaidis MG, Kyparos A, Kouretas D (2009) Blood reflects tissue oxidative stress depending on biomarker and tissue studied. Free Rad Bol Med 47: 1371-1374. Link: https://goo.gl/QfnmXa
- Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem 95: 351-8. Link: https://goo.gl/NW7ilR
- Omaye ST, Turnbull JD, Sauberlich HE (1979) Selected methods for determination of ascorbic acid in animal cells, tissues and fluids. In: McCormick DB, Wrigth LD, eds. Methods in Enzymology, Vitamins and Coenzymes 3–11. Link: https://goo.gl/HzHQbh
- Rice-Evans C, Diplock A, Symons C (1991) Techniques in Free Radical Research. In: Burdon RH, Knippenberg PH, eds. Laboratory techniques in biochemistry and molecular biology. Elsevier 227-229
- Karadag F, Kirdar S, Karul AB, Emel Ceylan (2008) The value of C-reactive protein as a marker of systemic inflammation in stable chronic pulmonary disease. Eur J Intern Med 19: 104-108. Link: https://goo.gl/Qg4oX5
- Dahl M, Nordestgaard BG (2009) Markers of early disease and prognosis in COPD. International Journal of COPD 4: 157-167. Link: https://goo.gl/QvRWem
- Hurst JR, Donaldson GC, Perera WR, Wilkinson TMA, Bilello JA, et al. (2006) Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 174: 867-874. Link: https://goo.gl/k4sZqi
- Kim V, Rogers TJ, Criner GJ (2008) New concepts in the pathobiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc 5: 478-485. Link: https://goo.gl/1O7evr
- Skyba P, Kluchova Z, Joppa P, Petrasova D, Tkacova R (2009) Nutritional status in relation to respiratory impairment and systemic inflammation in patients with acute exacerbations of COPD. Med Sci Monit 15: CR528-CR533. Link: https://goo.gl/ZT5UQ3
- Pinto-Plata VM, Müllerova H, Toso JF, Feudjo-Tepie M, Soriano JB, et al. (2006) C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax 61: 23-28. Link: https://goo.gl/nQSz8o
- Karadag F, Karul AB, Cildag O, Altun C, Gurgey O (2004) Determinants of BMI in patients with COPD. Respirology 9: 70-75. Link: https://goo.gl/pBSgeF
- Izquierdo JL, Almonacid C, Parra T, Pérez J (2006) Systemic and lung inflammation in 2 phenotypes of chronic obstructive pulmonary disease. Arch Bronconeumol 42: 332-337. Link: https://goo.gl/pHo13W
- Jones PW, Agusti AGN (2006) Outcomes and markers in the assessment of chronic obstructive pulmonary disease. Eur Respir J 27: 822-832. Link: https://goo.gl/QLmw97
- Louhelainen N, Myllärniemi, Rahman I, Kinnula VL (2008) Airway biomarkers of the oxidant burden in asthma and chronic obstructive pulmonary disease: current and future perspectives. International Journal of COPD 3: 585-603. Link: https://goo.gl/Z19jp0
- MacNee W (2013) Systemic inflammatory biomarkers and co-morbidities of chronic obstructive pulmonary disease. Ann Med 45: 291-300. Link: https://goo.gl/Ev00rJ
- MacNee W (2005) Pathogenesis of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2: 258-266. Link: https://goo.gl/KrK4JR
- Raghunath RR, Phadke MS (2006) Plasma oxidant-antioxidant status in different respiratory disorders. Indian J Clin Biochem 21: 161-164. Link: https://goo.gl/YH8FnQ
- Tuder RM, Zhen L, Cho CY, Taraseviciene-Stewart L, Kasahara Y, et al. (2003) Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am J Respir Cell Mol Biol 29: 88-97. Link: https://goo.gl/6V3CLs
- Fischer B, Pavlisko E, Voynow JA (2011) Pathogenic triad in COPD: oxidative stress, protease-antiprotease imbalance, and inflammation. International Journal of COPD 6: 413-421. Link: https://goo.gl/J3D1a3
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
