Archives of Pulmonology and Respiratory Care
Impact of Calorie Intakes on the Risk of Bronchopulmonary Dysplasia in Extremely Preterm Infants
Belal Alshaikh1*, Siddartha Buddhavarapu1, Albert Akierman1, Abhay Lodha1, Reginald Sauve1,2 and Amuchou Soraisham1
2Community Health Sciences, Calgary, AB, Canada
Cite this as
Alshaikh B, Buddhavarapu S, Akierman A, Lodha A, Sauve R, et al. (2017) Impact of Calorie Intakes on the Risk of Bronchopulmonary Dysplasia in Extremely Preterm Infants. Arch Pulmonol Respir Care 3(1): 020-024. DOI: 10.17352/aprc.000019Aim: To examine whether caloric intake during the first week of age influences the risk of Bronchopulmonary Dysplasia (BPD) in extremely preterm infants.
Methods: In this retrospective cohort study, all infants born with gestational age < 29 weeks over 30 months period were eligible for the study. Infants with major congenital anomalies and those who died before 36 weeks postmenstrual age were excluded. We compared the nutritional characteristics between infants with BPD and those with no BPD.
Results: Of the 262 eligible infants, 233 were included for the study. A total of 125 infants developed BPD. Infants with BPD were likely to receive higher fluid intake in the first 2 days of age compared to infants with no BPD. After adjusting for gestational age, severity of illness and PDA, higher daily calorie intake, especially higher protein intake was associated with a trend towards lower odds of BPD (OR= 0.91; 95% CI: 0.81-1.01). Delay in reaching full enteral feed was associated with increased odds of BPD (OR= 1.03; 95%CI: 1.00 - 1.06).
Conclusions: In preterm infants born at less than 29 weeks, provision of adequate calorie intake; particularly protein, in the first week of life may be associated with decreased risk of BPD.
Introduction
Bronchopulmonary Dysplasia (BPD) is the most common morbidity in extremely preterm infants [1]. The prevalence of BPD varies widely from 5.5% to 67% depending on gestational age and medical center [2-4]. The pathogenesis of BPD is multi-factorial and include severe prematurity, volutrauma or barotrauma, oxygen toxicity, perinatal infection and inflammation [5]. Excessive fluid intake in the early postnatal period has been proposed as an additional risk factor for BPD [6-8]. However, the evidence supporting this is still conflicting.
Extracellular fluid (ECF) represent a large proportion of body water content in neonate [9]. The ECF undergo a physiologic contraction in the first few days after birth allowing a negative fluid balance [10]. This results in weight loss in the early neonatal period. In order to achieve the appropriate weight loss, the fluid intake needs to be less than the amount of water excreted through the kidney or lost as insensible water [11]. This physiologic process may not occur if the preterm infants received excessive fluid after birth. In addition, studies have suggested that excessive fluid intake may result in higher incidence of symptomatic patent ductus arteriosus (PDA) [12]. Retention of ECF fluid in the presence of PDA with left-to-right shunt is suggested cause for pulmonary interstitial edema, prolonged mechanical ventilation, and subsequent BPD [13].
Nutrition is a critical factor influencing the growth of extremely preterm infants. Studies have shown that adequate nutrition may reduce the risk and severity of BPD [14,15]. However, providing adequate nutrition usually requires higher fluid intake particularly in the first few days of life which imposes the potential benefit of appropriate fluid intake.
The primary objective of this study was to identify whether increase fluid intake during the first two weeks of life is associated with increased risk of BPD in extremely preterm infants. In addition, we aim to describe the relationship between the type of calorie intake during this period and the rate of BPD. We primarily hypothesized that high fluid intake in the first two weeks of life is associated with increased risk for BPD in preterm infants < 29 weeks.
Patients and Methods
This retrospective cohort study was conducted at Foothills Medical Centre (FMC) in Calgary with approximately 7000 deliveries annually. This study was approved by the conjoint health research ethics board of University of Calgary. All preterm infants born with birth gestational age < 29 weeks and admitted to the FMC neonatal intensive care unit (NICU) between January 2008 and June 2010 were eligible for the study. Preterm infants with congenital anomalies were excluded from the study.
All charts of these infants were reviewed for the following data in the first 14 days of life: daily weight, daily total fluid intake, daily calorie intake, daily amount and type of feed, and daily weight loss. The mean daily protein, lipid and energy intake were calculated based on actual intake. Day 1 was defined as the first full 24 h period after birth. If the infant was on enteral feeds, the energy and protein intakes were calculated using preterm breast milk composition for the first (60 kcal/100 ml and 2.2 g protein/100 ml) and the second (71 kcal/100 ml and 1.5 g protein/100 ml) week of life [16]. The enteral feeds were initiated at the discretion of attending neonatologist guided by the unit’s enteral feeding protocol, which remained unchanged in both time periods. The trophic feed was started once the baby is hemodynamically stable and feeds were gradually advanced by 10–20 ml/kg/d according to the feeding protocol. Once the infant reached 100 ml/kg/d of enteral feeding, human milk fortifier was added to a maximum of 4 packed/100 ml of expressed human milk. Mother’s own milk was used in all but 2 infants who received preterm formula. Calculations in infants on formula or human milk fortifiers were performed using composition of these products as described by the manufacturer. Body weight was measured by the bedside registered nurse using electronic scales. Weight was done daily unless the infant is too sick to tolerate the procedure. In addition, maternal and neonatal demographics, clinical characteristics, and neonatal outcomes were collected.
Respiratory distress syndrome (RDS) was defined as the presence of respiratory signs including tachypnea, grunting and chest retraction, typical chest x-ray findings, and/or treatment by surfactant. BPD was defined as oxygen dependency at 36 weeks post-menstrual age (PMA) [17]. PDA was defined as clinical diagnosis confirmed by ECHO. Intraventricular hemorrhage (IVH) was defined according to the criteria of Papile et al., from head ultrasound [18]. Necrotizing enterocolitis (NEC) was defined according to modified Bell’s criteria (stage 2 or higher) [19].
Statistical analysis
Descriptive statistics including means, medians and standard deviations (SD) were used to describe the study population. In order to compare between continuous variables in infants with and without BPD, two sample t test or Mann-Whitney test were used as appropriate. These two tests were performed to compare between total and enteral volumes, maximum proportions of weight loss, and calorie intakes on days 1 to 14. Chi -square test was used to compare discrete variables unless the expected cell frequency was less than five then Fisher’s exact test was performed. The association between amount of fluid intake and the BPD was examined using multivariable logistic regression to adjust for potential confounding factors. In order to avoid collinearity, birth weight was not included in the models as it is a factor in the Score for Neonatal Acute physiology and Perinatal Extention ΙΙ (SNAPPE-ΙΙ). Results of the multivariable logistic regression were presented as Odds Ratios (ORs) with 95% confidence intervals (CIs). Significance for all tests was established at a P value of <0.05. All p values were based on two sided test results. Stata version 11.0 software (Stata Corporation, College Station, Texas, USA) was used for all data analyses.
Results
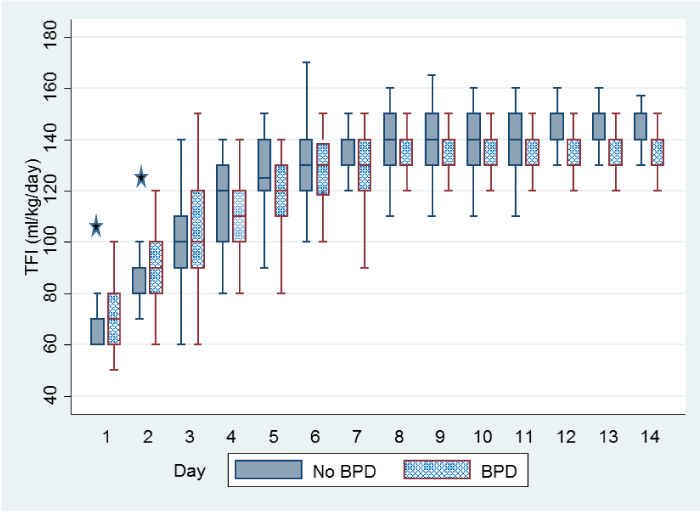
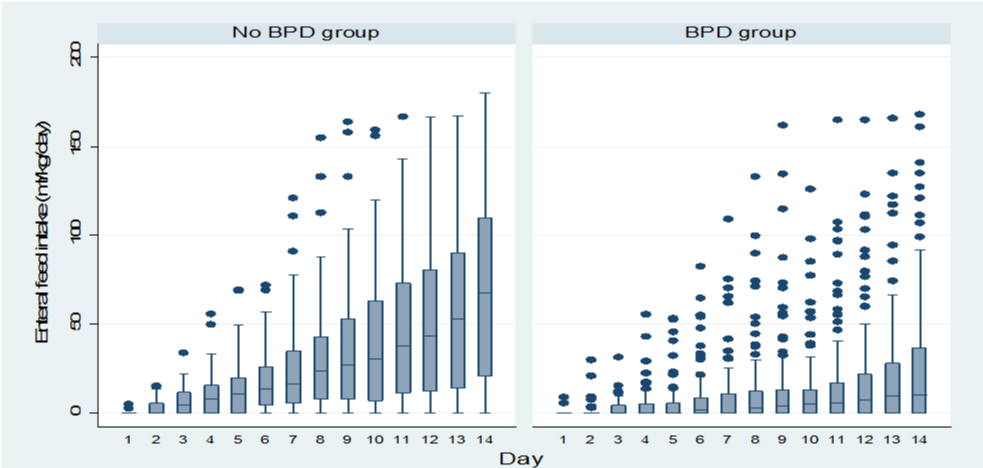
A total of 262 preterm infants with gestational age < 29 weeks were admitted during the study period. Nineteen infants died and other ten infants were excluded; five due to congenital anomalies and five due to missing data. Neonatal and maternal characteristics are summarized in table 1. Within the total cohort, one hundred and twenty five infants (53.6%) developed BPD. Compared to infants with no BPD, infants who developed BPD had significantly lower gestational age, lower birth weight, lower Apgar scores at 5-minute, higher SNAPPE-ΙΙ score, longer duration of assisted ventilation, and more use of postnatal steroids. In addition, these infants had higher prevalence of RDS, use of surfactant, PDA, NEC, and culture-proven sepsis. The policy of our NICU is to include the volume used for trophic feed in the total fluids. Figure 1 shows the amount of total fluids given to the infants in the two groups. Total fluid intake was significantly higher in the first 2 days of life in infants developed BPD. No difference was noted on day 3 and 4. After day 4, the total fluid intake became significantly less in infants who developed BPD. Volume of enteral feed was significantly lower in the first 2 weeks of life in infants who developed BPD (Figure 2).
Weight loss (defined as percentage of birth weight) in BPD infants was lower than those who survived without BPD. This loss was statistically significant in day 3, 4 and 5 (4% vs 6%, 6 vs 8%, 7% vs 9%, respectively). Infants in both groups had their nadir of weight loss by day 5. However infants who developed BPD regained their birth weight earlier than those without BPD.
The nutritional characteristics of the infants in our cohort are summarized in the table 2. The time of first enteral feed was later in infants who developed BPD. The time required to reach full feed (as defined by minimum 120 ml/kg/day of enteral feed) was more prolonged in infants who developed BPD. Similarly, the duration of parental nutrition was longer in infants who developed BPD. On the bivariate analysis, the mean total calorie intake per day in the first week of life was significantly higher in infants who survived without BPD. Additionally, the mean lipid intake per day during the first week was statistically higher in these infants.
After adjusting for gestational age, SNAPPE-ΙΙ, and PDA using logistic regression model, higher daily calorie intake, especially higher protein intake was associated with lower odds of BPD. Delay in reaching full enteral feed was associated with increase odds of BPD (Table 3).
Discussion
The results of this retrospective study suggest that lower calorie and particularly protein intake is associated with increased risk of BPD.
BPD is a multifactorial disease, however; it is primarily resulted from prematurity and lung injury. Mechanical ventilation, oxygen toxicity, and infection/inflammation have been always the keys to understand the pathogenesis of BPD [1,5]. Inappropriate high fluid intake in the first 3 days after birth is proposed to lower lung compliance during the retention of extracellular fluid phase. This may subsequently lead to additional needs for oxygen and respiratory support in order to maintain adequate ventilation and oxygenation.
Whether the increase in total body water of preterm infants; as commonly resulted from decreased renal losses in the first few days of life, or the excessive fluid therapy itself increases the risk of BPD has always been an issue of debate. Association between PDA and BPD has frequently described in the literature [20]. Oh et al., demonstrated similar association between excess fluid intake and risk of BPD or death [11]. Our study did not include death in the outcome because many infants died after the joint decision between parents and caregiver to withdrawal of care due to critical morbidity such as severe IVH. Van Marter et al., published similar results however they defined BPD as oxygen-dependence at 28 days of age [4]. Our study used the definition of BPD as oxygen-dependence at 36 weeks postmenstrual age [17].
A systematic review of 5 randomised trials by Bell and Acarregui indicates that restricted water intake significantly reduces the risks of PDA and NEC with a trend towards reduced risk of BPD [21]. The high fluid volume described in this study was > 169 ml/kg/day which unlikely to be achieved in the first few days of life in modern NICUs with the ability to maintain good humidity around the extreme preterm infants. Furthermore, three studies [12,22,23], in this review were included from the pre-surfactant and pre-antenatal steroid era. Our results indicated that there was an increased risk of BPD with increase in the fluid intake ion the first few days of life. The policy in our NICU is to keep high humidity (85-95%) in the first week of life in order to decrease insensible water loss for all preterm infants born < 29 weeks gestation. Therefore; the need for using very high fluid intake (>169 ml/kg/day) is almost eliminated.
In contrast, lower fluid intake may result in lower caloric intake. The recommended calorie intake for extremely preterm infants is 110 to 135 Kcal/kg/day which is usually achieved by the second week of life [24]. The initial basic parenteral need for non-growing preterm infants in the first week of life is estimated to be about 61 to 63 Kcal/kg/day in order to meet the resting basal metabolic rate and other non-growth related energy needs [8,24,25]. Our study demonstrated that infants who survived without BPD reached to their calorie goal earlier than those who developed BPD. In addition, survivors without BPD had significantly higher enteral intake in the first 2 weeks of life probably due to better feeding tolerance and more stable clinical course. This is similar to the findings reported by Oh et al. [11].
Low protein intake and subsequent hypoproteinemia may result in reduction of the osmotic pressure gradient [26]. This can cause a large transcapillary flux of fluid into the interstitial spaces of the lung, a reduction in the lung compliance, and probably a need for more respiratory support [26]. All factors increase the risk for BPD.
The maximum weight loss in infants who developed BPD was similar to those infants without BPD in our cohort; however the number of days needed to regain birth weight was significantly less in infants who developed BPD. In addition, the increase in weight loss in the first 2 weeks of life did not correlate with the reduction in the rate of BPD. This finding was consistent with other studies which clearly showed that excessive weight loss does not prevent BPD [12,22,27].
Other factors that influenced the risk of BPD in our study include PDA, culture-proven sepsis and male sex. These factors were frequently reported in the literature [28-31].
The strengths of our study are the large sample size, the ability to study the impact of fluid and calorie intakes simultaneously, and the ability to control for various potential confounding factors including the illness severity. As a retrospective observational study, several limitations exist include the lack of data on criteria of ventilation, supplemental oxygen and targeted oxygen saturation limit, daily sodium intake, and daily urine output.
Despite the limitations, our study has important clinical implications. First, it identifies role of early nutrition in protecting extremely preterm infants from developing BPD. Second, it stimulates the future research to determine the etiology of which some of these risk factors increase the risk for BPD. In conclusion, excessive fluid intake in premature infants in the first 3 days of life is associated with increased risk of BPD. Providing adequate calorie intake particularly adequate protein intake in the first week of life may help in reducing the incidence of BPD. Enteral feeding is commonly delayed; therefore health care providers are required to establish enteral feeding whenever that is possible and safe. Finally, vigilant attention should be paid to meet the calorie needs of these infants without liberalising their total fluid intake.
- Christou H, Brodsky D (2005) Lung injury and bronchopulmonary dysplasia in newborn infants. J Intensive Care Med 20: 76-87. Link: https://goo.gl/Y3xr0e
- Latini G, De Felice C, Giannuzzi R, Del Vecchio A (2013) Survival rate and prevalence of bronchopulmonary dysplasia in extremely low birth weight infants. Early Hum Dev 1: S69-73. Link: https://goo.gl/JVsh0t
- Farstad T, Bratlid D, Medbo S, Markestad T, Norwegian Extreme Prematurity Study G (2011) Bronchopulmonary dysplasia - prevalence, severity and predictive factors in a national cohort of extremely premature infants. Acta paediatrica 100: 53-58. Link: https://goo.gl/SnsXkO
- Van Marter LJ, Pagano M, Allred EN, Leviton A, Kuban KC (1992) Rate of bronchopulmonary dysplasia as a function of neonatal intensive care practices. J Pediatr 120: 938-946. Link: https://goo.gl/WXXB1y
- Bancalari E, Claure N, Sosenko IR (2003) Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Semin Neonatol 8: 63-71. Link: https://goo.gl/QbWCYW
- Van Marter LJ, Leviton A, Allred EN, Pagano M, Kuban KC (1990) Hydration during the first days of life and the risk of bronchopulmonary dysplasia in low birth weight infants. J Pediatr 116: 942-949. Link: https://goo.gl/7Rxqdu
- Marshall DD, Kotelchuck M, Young TE, Bose CL, Kruyer L, et al. (1999) Risk factors for chronic lung disease in the surfactant era: a North Carolina population-based study of very low birth weight infants. North Carolina Neonatologists Association. Pediatrics 104: 1345-1350. Link: https://goo.gl/x4tht8
- Stephens BE, Gargus RA, Walden RV, Mance M, Nye J, et al. Fluid regimens in the first week of life may increase risk of patent ductus arteriosus in extremely low birth weight infants. J Perinatol 28: 123-128. Link: https://goo.gl/XrpnGX
- Friis-Hansen B (1961) Body water compartments in children: changes during growth and related changes in body composition. Pediatrics 28: 169-181. Link: https://goo.gl/qVyd5Q
- Bauer K, Versmold H (1989) Postnatal weight loss in preterm neonates less than 1,500 g is due to isotonic dehydration of the extracellular volume. Acta paediatrica Scandinavica. Supplement 360: 37-42. Link: https://goo.gl/3zjypW
- Oh W, Poindexter BB, Perritt R, et al. Association between fluid intake and weight loss during the first ten days of life and risk of bronchopulmonary dysplasia in extremely low birth weight infants. J Pediatr 147: 786-790. Link: https://goo.gl/9QZF0b
- Bell EF, Warburton D, Stonestreet BS, Oh W (1980) Effect of fluid administration on the development of symptomatic patent ductus arteriosus and congestive heart failure in premature infants. N Engl J Med 302: 598-604. Link: https://goo.gl/CmbT4A
- Brown ER (1979) Increased risk of bronchopulmonary dysplasia in infants with patent ductus arteriosus. J Pediatr 95: 865-866. Link: https://goo.gl/UnAmzd
- Shah PS (2003) Current perspectives on the prevention and management of chronic lung disease in preterm infants. Paediatric drugs 5: 463-480. Link: https://goo.gl/pGssZH
- Sosenko IR, Frank L (1991) Nutritional influences on lung development and protection against chronic lung disease. Semin Perinatol 15: 462-468. Link: https://goo.gl/hJrgb9
- Gidrewicz DA, Fenton TR (2014) A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC pediatrics 14: 216. Link: https://goo.gl/Yk1dcl
- Jobe AH, Bancalari E (2001) Bronchopulmonary dysplasia. American journal of respiratory and critical care medicine 163: 1723-1729. Link: https://goo.gl/EzPmkN
- Papile LA, Munsick-Bruno G, Schaefer A (1983) Relationship of cerebral intraventricular hemorrhage and early childhood neurologic handicaps. The Journal of pediatrics 103: 273-277. Link: https://goo.gl/BxXRdL
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, et al. (1978) Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of surgery 187: 1-7. Link: https://goo.gl/T8i31R
- Clyman RI (2013) The role of patent ductus arteriosus and its treatments in the development of bronchopulmonary dysplasia. Semin Perinatol 37: 102-107. Link: https://goo.gl/Wq1OYe
- Bell EF, Acarregui MJ (2008) Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev 1: CD000503. Link: https://goo.gl/xnVo7t
- Lorenz JM, Kleinman LI, Kotagal UR, Reller MD (1982) Water balance in very low-birth-weight infants: relationship to water and sodium intake and effect on outcome. The Journal of pediatrics J Pediatr 101: 423-432. Link: https://goo.gl/rgMNYA
- von Stockhausen HB, Struve M (1980) [Effects of highly varying parenteral fluid intakes in premature and newborn infants during the first three days of life (author's transl)]. Klinische Padiatrie 192: 539-546. Link: https://goo.gl/VDfmF9
- Civardi E, Tzialla C, Garofoli F, Mazzucchelli I, Bollani L, et al. (2011) Nutritional needs of premature infants. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 1:27-29. Link: https://goo.gl/dtXqmn
- Neu J (2007) Gastrointestinal development and meeting the nutritional needs of premature infants. Am J Clin Nutr 85: 629S-634S. Link: https://goo.gl/FIC4RV
- Scallan J, Huxley VH, Korthuis RJ (2010) Capillary Fluid Exchange: Regulation, Functions, and Pathology. San Rafael (CA). Link: https://goo.gl/dnjaIB
- Tammela OK, Koivisto ME (1992) Fluid restriction for preventing bronchopulmonary dysplasia? Reduced fluid intake during the first weeks of life improves the outcome of low-birth-weight infants. Acta paediatrica 81: 207-212. Link: https://goo.gl/LA3mxa
- Hernandez-Ronquillo L, Tellez-Zenteno JF, Weder-Cisneros N, Salinas-Ramirez V, Zapata-Pallagi JA, et al. (2004) Risk factors for the development of bronchopulmonary dysplasia: a case-control study. Arch Med Res 35: 549-553. Link: https://goo.gl/45TgkT
- Korhonen P, Tammela O, Koivisto AM, Laippala P, Ikonen S (1999) Frequency and risk factors in bronchopulmonary dysplasia in a cohort of very low birth weight infants. Early human development 54: 245-258. Link: https://goo.gl/CP51Jl
- Zhang H, Fang J, Su H, Chen M (2011) Risk factors for bronchopulmonary dysplasia in neonates born at </= 1500 g (1999-2009). Pediatr Int 53: 915-920. Link: https://goo.gl/zqZwUt
- Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med 183: 1715-1722. Link: https://goo.gl/wwi3qD
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
