Archives of Pulmonology and Respiratory Care
Mucin Production Correlates with Dual Expression of Epidermal Growth Factor Receptor and Its Ligand the Epidermal Growth Factor in Non-Small Cell Lung Cancer
1Department of Pathology, Manuel Fajardo General Hospital, Zapata and D Street Vedado, Plaza de la Revolución, 10400 Havana, Cuba
2Laboratory of Biochemistry, Center of Molecular Immunology, 216 Street and 15 Avenue, Atabey, Playa, PO Box 16040, 11600 Havana, Cuba
3Department of Cell Biology and Tissues Banking, National Institute of Oncology and Radiobiology, 10400 Havana, Cuba
Author and article information
Cite this as
Blanco R, Rengifo CE, Domínguez E, Blanco D, Cedeño M, et al. (2017) Mucin Production Correlates with Dual Expression of Epidermal Growth Factor Receptor and Its Ligand the Epidermal Growth Factor in Non-Small Cell Lung Cancer. Arch Pulmonol Respir Care. 2017; 3(1): 025-031. Available from: 10.17352/aprc.000020
Copyright License
© 2017 Blanco R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction: Mucoproduction plays an important role in the processes of tumor progression, invasion and metastasis. In non-small cell lung cancer (NSCLC), mucins hypersecretion has been associated with alterations in the epidermal growth factor receptor (EGFR) expression. However, the relation of mucins with EGFR in these tumors is not completely clarified.
Aims: To evaluate the relation between mucoproduction, the expression of EGFR and its ligand the epidermal growth factor (EGF) in NSCLC samples.
Materials and Methods: A number of 71 routinely processed formalin-fixed and paraffin-embedded archival samples with diagnosis of NSCLC were used. Mucoproduction was considered when periodic acid-Schiff reaction (PAS) positive and/or alcian blue positive substances were observed. The immunolocalization of EGFR and EGF molecules were detected by mean of immunohistochemical methods. Chi-square test was applied to compare two or three different parameters. In all cases, the criterion for statistical significance was p<0.05.
Results: The production of mucins was related with the occurrence of metastasis (p = 0.0151) as well as, with a more advanced stage of NSCLC disease (p = 0.0409). The presence of mucin was statistically significant associated to histology (p < 0.0001) and degree of cytological atypia (p = 0.0325). The expression of EGFR was associated with mucin hyperproduction (p = 0.0205). The levels of mucin production was statistical significant increase in NSCLC samples displaying double expression of EGFR and EGF (p=0.0297) when compared with EGFR-/EGF+ and EGFR-/EGF-.
Conclusions: The results obtained in the present work could support the role of uncontrolled EGFR system activation mediated by the ligand EGF in NSCLC mucins hyperproduction as well as its relation with tumor progression and metastasis processes.
Abbreviations
AB: Alcian Blue Stain; ABC: Avidin Biotin Complex; BAC: Bronchioloalveolar Carcinoma; DAB: 3,3-Diaminobenzidine; EGF: Epidermal Growth Factor; EGFR: Epidermal Growth; Factor Receptor; HE: Hematoxylin And Eosin Staining; HRP: Horse-Radish Peroxidase; Mab: Monoclonal Antibody; MAPK: Mitogen-Activated Protein Kinase; L MUC: Mucin; NSCLC: Non-Small-Cell Lung Cancer; PAS: Periodic Acid-Schiff Stain; SCLC: Small-Cell Lung Cancer
Introduction
Lung cancer remains one of the leading causes of death from cancer worldwide [1]. There are two main variant of the disease, non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC), but, NSCLC constitute about 80-85% cases of all lung carcinomas [2,3]. Most of NSCLC patients have a poor prognosis because most of them present with advanced or metastatic disease at the time of diagnosis. It has been estimated that only 10-15% of the patients will ultimately be cured [4].
Mucins constitute a heterogeneous group of highly O-glycosylated macromolecules synthesized by epithelial cells [5]. Airway mucins are major components of the soluble layer and/or viscoelastic gel that comprise lung mucus in healthy airways and contribute to the mucociliary defense system that protects the lungs against pathogens and environmental toxins [6]. Particularly, increased hyperproduction of secretory mucins usually occurs in lung tumors [5,7]. Nevertheless, their cellular localization and variations along the respiratory tract or occurring in respiratory diseases have received little attention.
Mucoproduction plays an important role in the processes of tumor progression, invasion and metastasis. In addition, mucins are engaged in malignant cells survival and protection against the host immune response [8], contributing to the aggressive potential of tumors. Airway obstruction from mucus accumulation is one of the major pathological changes in NSCLC [9]. In line with this, chronic mucus hypersecretion has been considered a significant predictor of death from these tumors [10]. However, further investigations in order to elucidate the biological significance of mucins in carcinomas have been suggested [11].
Previously, the epidermal growth factor receptor (EGFR) mutation was considered a critical event in the pathogenesis of nonmucinous bronchioloalveolar carcinoma (BAC) [12] while, invasive mucinous pattern and extracellular mucin have been associated with KRAS mutation in lung adenocarcinoma [13]. Moreover, the lepidic-predominant group was associated with EGFR mutation compared with nonlepidic-predominant tumors [13]. Furthermore, one preliminary study demonstrated a significant correlation between nonmucinous BAC histology and EGFR expression [14]. Nevertheless, the relation of mucins with EGFR expression in lung cancer is not complete clarified [7].
In this study, it was evaluated the relation between mucoproduction and the expression of EGFR in NSCLC patients. Additionally, it was assessed the role of the double expression of EGFR and its ligand the EGF in mucins hyperproduction.
Materials and Methods
Tissue specimens. A number of 71 routinely processed formalin-fixed and paraffin-embedded archival samples with diagnosis of NSCLC were received from the pathology department of both Hermanos Ameijeiras General Hospital and the National Institute of Oncology and Radiobiology, after approved consent by the institutional ethical committees. Five micrometer serial sections from each block were obtained in a micrometer (Leitz 1512) and they were mounted on plus slides (Dako S2024). All sections were attached to the slide by heating in a 60ºC oven for 1h. Afterwards, the slides were dewaxed in xylene and rehydrated in graded ethanol series in the usual way. The samples were maintained in tap water until they were stained.
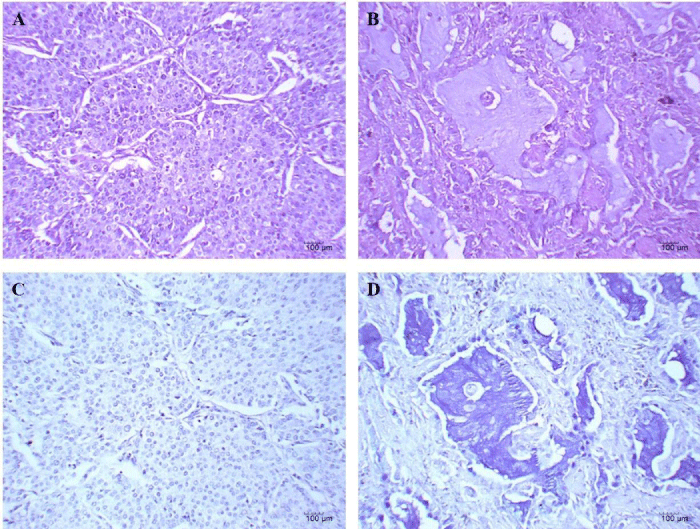
Pathological features. The evaluation of some pathological features was performed for each tumor tissues using the hematoxylin and eosin (HE) staining. Morphologic parameters such as histopathological classification and degree of histologic differentiation were evaluated. In addition, some cytomorphologic characteristics such as cell and nuclear size, cellular shape, chromatin pattern, nucleoli, and amount of cytoplasm were assessed. This measured was considered as the degree of cytological atypia and it was subjectively expressed as low, moderate and high.
Mucoproduction detection. The Alcian blue pH 2.5-P.A.S. acc. Mowry kit (DiaPath 010209) according to the manufacturer instructions was used. Briefly, the slides were stained with Alcian Blue (pH 2.5) for 30 minutes at room temperature. The samples were drained and treated with a saturated solution of sodium tetraborate for 10 minutes. After two rinse in running tap and distilled water, the tissues were oxidized for 5 minutes in 1% periodic acid solution. Then, the slides were rinsed in several changes of distilled water and immersed in Schiff reagent acc. Hotchkiss-McManus for 30 minutes. Afterward, sections were dipped in a solution containing 90 mL of destilled water and 10 drops of metabisolfite potassium acc. Hotchkiss-McManus and 10 drops of 10% hydrochloric acid for 10 minutes. The sections were washed for 1 minute in distilled water. Finally, the tissues were counterstained with Mayer Hematoxylin, dehydrated in ethanol, cleared in xylene and mounted with a synthetic medium.
Immunohistochemical staining. The immunolocalization of the epidermal growth factor receptor (EGFR) and its ligand the epidermal growth factor (EGF) was performed as it was previously described in [15]. Briefly, the slides were pre-treated with 0.4% pepsin in 0.1N hydrochloric acid solution at 37°C for 30 minutes. Afterward, the tissues were incubated with ior egf/r3 Mab (anti-EGFR) and CB-EGF1 (anti-EGF ligand) in a humid chamber for 1h at room temperature followed by a rabbit anti-mouse biotinylated secondary antibody (Dako E0354) and ABC/HRP (Dako E0355) both for 30 minutes at room temperature dilution 1:100. The enzymatic activity was visualized with DAB substrate chromogenic solution (Dako K3465) and the tissues were counterstained with Mayer´s Hematoxylin (Dako S2020). Finally, the samples were dehydrated in ethanol, cleared in xylene and mounted with a synthetic medium.
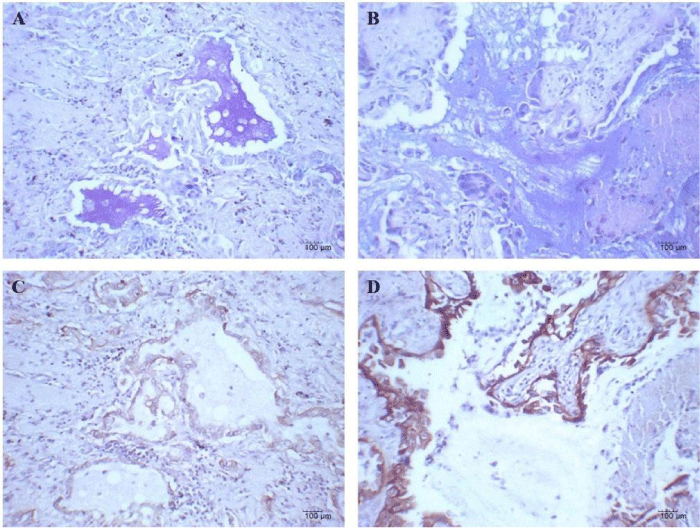
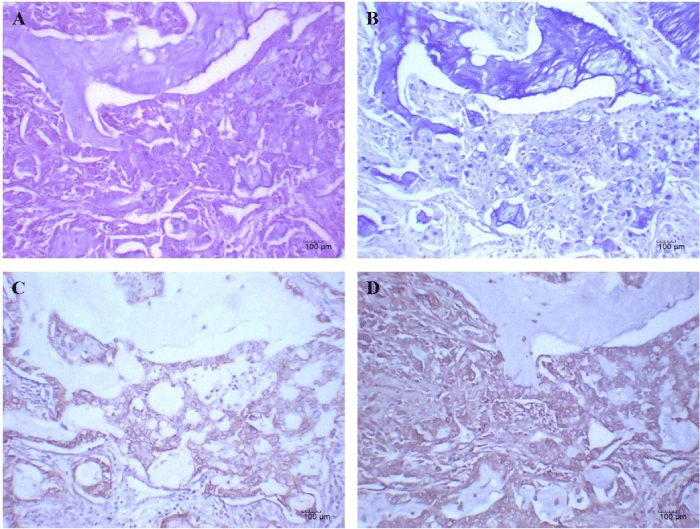
Histochemical and immunohistochemical evaluation. The Alcian blue pH 2.5-PAS acc. Mowry kit supplies reagents for Alcian blue (pH 2.5) and periodic acid-Schiff stains (AB-PAS) to identify on the same histological section acid and neutral mucins, glycogen and glycoprotein. In this study, the appearance of blue-turquoise and/or purple staining in both extra and intra-cellular locations was considered as histochemical detection of mucins.
Concerning to the immunohistochemistry, the tissue expression of the EGFR and EGF was evaluated for percentage of positive cells (0-100%) and the intensity of reaction (0-3+). The final results were considered according with two observers agreement (ChER and RB). Afterward, the H-score was calculated for each specimen by multiplication of the intensity of reaction and the grade of positive cells, resulting in a score ranging from 0 to 300. Subsequently, these scores were grouped as follow: low expression (scores < 150) and high expression (scores ≥ 150) as it was previously described in [16] (Table 1).
Statistical analysis. PopTools version 3.0.5 software (Available on: https://www.cse.csiro.au/poptools) was used for data analysis. Chi-square test was applied to compare two or three different parameters. In all cases, the criterion for statistical significance was p < 0.05.
Results
Patients characteristics. Table 1 shows a summary of patient characteristics and some pathological features. In general, the gender ratio was to 2:1 in favor of males, with a mean age of 57.2 ± 10.3 years. In addition, mostly patients in stages I-II (78.9%) and samples showing histological features of well and moderately differentiated tumors (67.6%) were included.
Mucins expression is associated with both occurrence of distant metastasis and advanced disease. In general, the mucin expression was observed in 35/71 (49.3%) of NSCLC. The mucoproduction was mainly detected in acinar structures formed by many tumor cells; although vacuoles in the interior of individual epithelial cells were also visualized. The production of mucins was significantly increased in patients displaying distant metastasis 7/7 (100%) when compared with no metastatic disease 28/64 (43.7%) (p = 0.0151, Chi-Square test). Similarity, the presence of mucoproduction was mostly evidenced in advanced stages of NSCLC disease 11/15 (73.3%) when compared with early stages 24/56 (42.9%) (p = 0.0409, Chi-Square test).
Mucins expression is also related with histology and degree of cytological atypia. The distribution of mucin production according to the histopathological classification of NSCLC is shown in Table 2. The presence of mucin was statistically significant increased in adenocarcinomas and other minor represented subtypes when compared with the rest of histological subtypes of NSCLC (p < 0.0001, Chi-Square test). In addition, the mucoproduction was significantly increased in samples with higher degree of cytological atypia (p = 0.0325, Chi-Square test) (Figure 1). No association between mucin hyperproduction and the rest of clinicopathological characteristics of patients were evidenced.
Mucin hyperproduction is related with EGFR expression. The expression of EGFR was detected in 42/71 (59.1%) of NSCLC samples no taking into account the histological classification of tumors. Regarding the histological classification of tumors, the EGFR expression was evidenced in 10/21 (47.6%) of squamous carcinoma, 18/27 (66.7%) of adenocarcinoma, 4/10 (40.0%) of large cell carcinoma, 5/8 (62.5%) of carcinoid tumor and 5/5 (100%) of other histological subtypes minor represented. No association between EGFR expression and histological subtype of tumors were detected. Of interest, the mucin production was evidenced in 26/42 (61.9%) of these EGFR positive cases Table 3. Consequently, the expression of EGFR was associated with mucin hyperproduction (p = 0.0205, Chi-Square test) (Figure 2).
26/42 (61.9%) of EGFR+/EGF+, 5/19 (26.3%) of EGFR-/EGF+ and 4/10 (40.0%) of EGFR-/EGF- samples Table 3. Consequently, the levels of mucin production was statistical significant increased in NSCLC samples displaying double expression of EGFR and its ligand the EGF (p = 0.0297, Chi-Square test) (Figure 3).
Discussion
Mucins have been implicated in the pathogenesis of epithelial cell malignancies [17]. Alterations of the expression pattern of mucins have been described in carcinomas as well as in their precursor lesions [18,19]. Airway obstruction from mucus accumulation or from a tumor projecting into a bronchus is one of the major pathological changes in lung cancer [9]. Specifically, chronic mucus hypersecretion has been considered a significant predictor of death from lung cancer [10]. Mucinous carcinoma has been known to have a propensity for higher incidence of lymph node metastasis, venous and lymphatic invasions, local recurrence and distant metastasis compared with nonmucinous tumors [20].
The detection of certain type of mucins (MUC) by means of immunohistochemical methods has been reported to be associated with the progression of lung adenocarcinoma [21]. In a previous work, our group demonstrated that PAS-positive substances correlated with the appearance of distant metastasis in NSCLC patients [22]. Here, the occurrence of mucin production was related with the appearance of distant metastasis but also with a more advanced disease. In line with this, Yu et al., reported that MUC5B and MUC5AC overexpressing tumors tended to increase the risk of distant metastasis and decrease overall survival from lung cancer, compared with nonexpressing tumors [23]. Moreover, aberrant MUC1 expression was associated with disease progression [24] and with a poor prognosis [25,26] while, up-regulation of MUC6 expression was related with tumor size in NSCLC patients [21].
In the present study, the mucin production was mostly evidenced in adenocarcinomas as compared with the rest of lung tumors, similar to previous reports [22,27,28]. Interestingly, the presence of mucin was significantly higher in NSCLC samples displaying increased degree of cytological atypia. Previously, it was published that transmembrane mucins (e.g MUC1 and MUC4), can signal the disruption of tight junctions and adherens junctions affecting polarity as well as both cell-cell and cell-extracellular matrix interactions [19,29]. It have been also reported that overexpression of MUC1 in cancer cells alter the function of β-catenin/E-cadherin pathway in adherens junctions [30] inducing actin cytoskeleton alterations [31]. In this sense, the loss of polarity and cell-cell interactions could alter the cell morphology allowing to an increased cell pleomorfism and also favors the development of metastasis [32].
EGFR cascade is involved in mucin production as well as in the repair of damaged airway epithelium by a wide variety of stimuli [33]. Changes that occur during lung carcinogenesis usually conduce to dys-regulation of both mucin and EGFR expressions [5,34]. In this study, the expression of EGFR was associated with mucoproduction measured by means of AB-PAS histochemical technique in NSCLC samples. Mao et al., reported a positive correlation between the expression of EGFR and MUC5AC in patients with chronic obstructive pulmonary disease [35]. However, in previous studies the EGFR mutation was related with nonmucinous bronchioloalveolar carcinoma [12,14] as well as with the lepidic-predominant form of this tumor [13].
On the other hand, the contribution of EGFR and its ligands in mucin production in normal airway epithelium using AB-PAS reaction was previously reported [36]. Co-expression of EGFR and its ligands results in activation of an autocrine system leading to dysregulated EGFR action and uncontrolled both tumor growth [37] and secretion [38]. It is known that mucins expression (e.g. MUC2 and MUC5AC) is controlled by EGF family and mitogen-activated protein kinase (MAPK) cascade [38]. Moreover, it has been reported that upregulated expression of mucins, such as MUC2 and MUC5AC, bind with EGFR and activate the Ras/Raf pathway [38,39]. In line with this, our group previously reported that the dual expression of EGFR and its ligand the EGF is related with increased cell proliferation and with a more aggressive behavior of NSCLC [16].
Interestingly, using selective inhibitors of EGFR tyrosine kinase phosphorylation, it was reported that EGFR ligand induced mucin MUC5AC synthesis is dependent on EGFR activation [36]. In this work, NSCLC samples displaying the phenotype EGFR+/EGF+ were associated with a more accentuated production of mucins as compared with EGFR+/EGF- and EGFR-/EGF- tumors. In line with this, EGFR phosphorylation upon binding to EGF and TGF-α resulted in a remarkable increase of MUC2 and MUC5AC mRNAs levels, promoter activity, and apomucin expression in the mucoepidermoid NCI-H292 lung cancer cell line [38]. In this sense, our results could support the role of uncontrolled EGFR system activation mediated by the ligand EGF in NSCLC mucins hyperproduction.
In view of that, it was suggested that disrupting the EGFR cascade that leads to mucus production is beneficial in airway hypersecretory diseases [40]. Interestingly, there are two different immunotherapeutic approaches registered in Cuba targeting the EGFR/EGF system. The first one is a molecular vaccine that induces anti-EGF antibodies neutralizing endogenous EGF (CIMAVax-EGF) [41]. The other one is a humanized monoclonal antibody that directly binds the extracellular domain of the EGFR (nimotuzumab) [42]. Interestingly, promising results in advanced NSCLC patients treated with CIMAVax-EGF [43,44] or nimotuzumab [45,46] has been obtained. In this way, the evaluation of mucin production in NSCLC patients after CIMAVax-EGF or nimotuzumab treatment seems to be of utmost importance.
Conclusions
The association between mucin hyperproduction and a more advanced state of NSCLC disease was demonstrated. Interestingly, mucoproduction was also related with the EGFR as well as with the double expression of EGFR and its ligand the EGF. Our results could suggest the role of mucins production mediated by EGFR/EGF stimulation in the aggressiveness of NSCLC. However, to confirm the results obtained in the present work, further studies eliminating differences in the proportion of histological subtypes and disease stages are needed. Remarkably, promising results were obtained in unresectable stage III NSCLC patients treated with an anti-MUC1 immunotherapeutic [47]. In this sense, our data permit to consider that immunotherapeutic strategies targeting EGFR and/or EGF could be a useful tools in the treatment of hypersecretory NSCLC.
Financial support was provided by the Center or Molecular Immunology.
Authors’ contribution
Rancés Blanco and Charles E. Rengifo authors contributed equally to this work.
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136: E359-386. Link: https://goo.gl/X2KoFI
- Giaccone G, Zucali PA (2008) Src as a potential therapeutic target in non-small-cell lung cancer. Ann Oncol 19: 1219-1223. Link: https://goo.gl/femwYy
- Barzi A, Pennell NA (2010) Targeting angiogenesis in non-small cell lung cancer: agents in practice and clinical development. EJCMO 2: 31-42. Link: https://goo.gl/SZueDo
- Berghmans T, Paesmans M, Sculier JP (2011) Prognostic factors in stage III non-small cell lung cancer: a review of conventional, metabolic and new biological variables. Ther Adv Med Oncol 3: 127-138. Link: https://goo.gl/jacdnV
- Copin MC, Devisme L, Buisine MP, Marquette CH, Wurtz A, et al. (2000) From normal respiratory mucosa to epidermoid carcinoma: expression of human mucin genes. Int J Cancer 86:162-168. Link: https://goo.gl/n2jmtG
- Rose MC, Voynow JA (2006) Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 86: 245-278. Link: https://goo.gl/6L89UT
- Lakshmanan I, Ponnusamy MP, Macha MA, Haridas D, Majhi PD, et al. (2015) Mucins in lung cancer: diagnostic, prognostic, and therapeutic implications. J Thorac Oncol 10: 19-27. Link: https://goo.gl/uQmS68
- Komatsu M, Yee L, Carraway KL (1999) Overexpression of sialomucin complex, a rat homologue of MUC4, inhibits tumor killing by lymphokine-activated killer cells. Cancer Res 59: 2229-2236. Link: https://goo.gl/whzKjp
- Des Jardins T, Burton GG (2016) Cancer of the Lung. In Clinical Manifestations and Assessment of Respiratory Disease. 7th edition. St. Louis, Mo: Mosby, 374-394. Link: https://goo.gl/w7yoKi
- Lange P, Nyboe J, Appleyard M, Jensen G, Schnohr P (1990) Ventilatory function and chronic mucus hypersecretion as predictors of death from lung cancer. Am Rev Respir Dis 141: 613-617. Link: https://goo.gl/sJMr3M
- Usman Ali, Nagi AH, Naseem N, Ullah E (2012) Mucin Histochemistry in Tumours of Colon, Ovaries and Lung. J Cytol Histol 3:163. Link: https://goo.gl/esVNhf
- Sakuma Y, Matsukuma S, Yoshihara M, Nakamura Y, Noda K, et al. (2007) Distinctive Evaluation of Nonmucinous and Mucinous Subtypes of Bronchioloalveolar Carcinomas in EGFR and K-ras Gene-Mutation Analyses for Japanese Lung Adenocarcinomas. Am J Clin Pathol 128: 100-108. Link: https://goo.gl/IJxB3p
- Kadota K, Yeh YC, D’Angelo SP, Moreira AL, Kuk D, et al. (2014) Associations between mutations and histologic patterns of mucin in lung adenocarcinoma: invasive mucinous pattern and extracellular mucin are associated with KRAS mutation. Am J Surg Pathol 38: 1118-1127. Link: https://goo.gl/bZpznS
- Franklin WA, Gumerlock PH, Crowley J, Chansky K, West HJ, et al. (2003) EGFR, HER2 and ERB-B pathway activation in bronchioloalveolar carcinoma (BAC): Analysis of SWOG 9417 and lung SPORE tissue samples. Proc Am Soc Clin Oncol 22: 620.
- Rengifo CE, Blanco R, Blanco D, Cedeño M, Frómeta M, et al. (2013) Immunohistochemical Characterization of Three Monoclonal Antibodies Raised against the Epidermal Growth Factor and Its Receptor in Non-Small-Cell Lung Cancer: Their Potential Use in the Selection of Patients for Immunotherapy. J Biomark 2013: 627845. Link: https://goo.gl/8TqqZF
- Blanco R, Domínguez E, Morales O, Blanco D, Martínez D, et al. (2015) Prognostic significance of N-Glycolyl GM3 ganglioside expression in non-small cell lung carcinoma patients: new evidences. Patholog Res Int 2015: 132326. Link: https://goo.gl/BNlRht
- Hollingsworth MA, Swanson BJ (2004) Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer 4: 45-60. Link: https://goo.gl/Bc5YL3
- Singh AP, Moniaux N, Chauhan SC, Meza JL, Batra SK (2004) Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res 64: 622-630. Link: https://goo.gl/KBsEjD
- Kufe DW (2009) Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer 9: 874-885. Link: https://goo.gl/1NOZTd
- Yamamoto S, Mochizuki H, Hase K (1993) Assessment of clinicopathologic features of colorectal mucinous adenocarcinoma. Am J Surg 166: 257-261. Link: https://goo.gl/J4f8ba
- Awaya H, Takeshima Y, Yamasaki M, Inai K (2004) Expression of MUC1, MUC2, MUC5AC, and MUC6 in Atypical Adenomatous Hyperplasia, Bronchioloalveolar Carcinoma, Adenocarcinoma With Mixed Subtypes, and Mucinous Bronchioloalveolar Carcinoma of the Lung. Am J Clin Pathol 121: 644-653. Link: https://goo.gl/ReKXRK
- Blanco R, Rengifo ChE, Cedeño M, Reyes K, Frómeta M, et al. (2015) Histochemical detection of carbohydrates in non-small cell carcinoma sections: its association with some clinic-pathological features. JOBARI 3: 9-17. Link: https://goo.gl/UZbQgZ
- Yu CJ, Shih JY, Lee YC, Shun CT, Yuan A, et al (2005) Sialyl Lewis antigens: association with MUC5AC protein and correlation with post-operative recurrence of non-small cell lung cancer. Lung Cancer 47: 59-67. Link: https://goo.gl/ECuJJJ
- Demirag F, Cakir E, Bayiz H, Yazici UE. (2013) MUC1 and bcl-2 expression in preinvasive lesions and adenosquamous carcinoma of the lung. Acta Chir Belg 113: 19-24. Link: https://goo.gl/yod1YP
- Nagai S, Takenaka K, Sonobe M, Ogawa E, Wada H, et al. (2006) A novel classification of MUC1 expression is correlated with tumor differentiation and postoperative prognosis in non-small cell lung cancer. J Thorac Oncol 1: 46-51. Link: https://goo.gl/bd2ul9
- Situ D, Wang J, Ma Y, Zhu Z, Hu Y, et al. (2011) Expression and prognostic relevance of MUC1 in stage IB non-small cell lung cancer. Med Oncol 28: 596-604. Link: https://goo.gl/aEFSOX
- Ocque R, Tochigi N, Ohori NP, Dacic S (2011) Usefulness of Immunohistochemical and Histochemical Studies in the Classification of Lung Adenocarcinoma and Squamous Cell Carcinoma in Cytologic Specimens. Am J Clin Pathol 136: 81-87. Link: https://goo.gl/3zux6s
- Dubinski W, Leighl NB, Tsao MS, Hwang DM (2012) Ancillary Testing in Lung Cancer Diagnosis. Pulm Med 2012: 249082. Link: https://goo.gl/9uTa3O
- Komatsu M, Carraway C, Fregien NL, Carraway KL (1997) Reversible disruption of cell-matrix and cell-cell interactions by overexpression of sialomucin complex. J Biol Chem 272: 33245-3354. Link: https://goo.gl/33Iiiq
- Yamamoto M, Bharti A, Li Y, Kufe D (1997) Interaction of the DF3/MUC1 breast carcinoma-associated antigen and β-catenin in cell adhesion. J Biol Chem 272: 12492-12494. Link: https://goo.gl/uL9Xz0
- Nelson WJ (2008) Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans 36: 149-155. Link: https://goo.gl/eHkeHU
- Komatsu M, Tatum L, Altman NH, Carothers Carraway CA, Carraway KL (2000) Potentiation of metastasis by cell surface sialomucin complex (rat MUC4), a multifunctional anti-adhesive glycoprotein. Int J Cancer 87: 480-486. Link: https://goo.gl/D7RuH8
- Puddicombe SM, Polosa R, Richter A, Krishna MT, Howarth PH, et al. (2000) Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J 14: 1362-1374. Link: https://goo.gl/EGAC8v
- Piythilake CJ, Frost AR, Manne U, Weiss H, Bell WC, et al. (2002) Differential expression of growth factors in squamous cell carcinoma and precancerous lesions of the lung. Clin Cancer Res 8: 734-744. Link: https://goo.gl/OHp93D
- Mao L, Bai CX, Zhang M, Wang YH, Chen J (2004) Expression of epidermal growth factor receptor and MUC5AC on human airway with chronic obstructive pulmonary disease. Chinese journal of tuberculosis and respiratory diseases 27: 585-588. Link: https://goo.gl/U6nEzB
- Takeyama K, Dabbagh K, Lee HM, Agustí C, Lausier JA, et al. (1999) Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci USA 96: 3081-3086. Link: https://goo.gl/DdCk0h
- Arteaga CL (2002) Epidermal Growth Factor Receptor Dependence in Human Tumors: More Than Just Expression? Oncologist 7: 31-39. Link: https://goo.gl/mXujXl
- Perrais M, Pigny P, Copin MC, Aubert JP, Van Seuningen I (2002) Induction of MUC2 and MUC5AC mucins by factors of the epidermal growth factor (EGF) family is mediated by EGF receptor/Ras/Raf/extracellular signal-regulated kinase cascade and Sp1. J Biol Chem 277: 32258-32267. Link: https://goo.gl/M2orKH
- Kim S, Lewis C, Nadel JA (2011) CCL20/CCR6 feedback exaggerates epidermal growth factor receptor-dependent MUC5AC mucin production in human airway epithelial (NCI-H292) cells. J Immunol 186: 3392-3400. Link: https://goo.gl/mr7AKV
- Burgel PR, Nadel JA (2004) Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium. Thorax 59: 992-996. Link: https://goo.gl/fcVt3E
- Gonzalez G, Crombet T, Catala M, Mirabal V, Hernandez JG, et al. (1998) A novel cancer vaccine composed of human-recombinant epidermal growth factor linked to a carrier protein: Report of a pilot clinical trial. Ann Oncol 9: 431-435. Link: https://goo.gl/EHy6NQ
- Crombet-Ramos T, Rak J, Perez R, Viloria-Petit A (2002) Antiproliferative, antiangiogenic and proapoptotic activity of h-R3: A humanized anti-EGFR antibody. Int J Cancer 101: 567-575. https://goo.gl/D0L9SN
- García B, Neninger E, de la Torre A, Leonard I, Martínez R, et al. (2008) Effective inhibition of the epidermal growth factor/epidermal growth factor receptor binding by anti-epidermal growth factor antibodies is related to better survival in advanced non–small-cell lung cancer patients treated with the epidermal growth factor cancer vaccine. Clin Cancer Res 14: 840-846. Link: https://goo.gl/XRYSjV
- Neninger Vinageras E, de la Torre A, Osorio Rodríguez M, Catalá Ferrer M, Bravo I, et al. (2008) Phase II randomized controlled trial of an epidermal growth factor vaccine in advanced non-small-cell lung cancer. J Clin Oncol 26: 1452-1458. Link: https://goo.gl/7Ock7i
- Choi HJ, Sohn JH, Lee CG, Shim HS, Lee IJ, et al. (2010) A phase I study of nimotuzumab in combination with radiotherapy in stages IIB–IV non-small cell lung cancer unsuitable for radical therapy: Korean results. Lung Cancer 71: 55-59. Link: https://goo.gl/qyhdrn
- Wang HQ, Ren Y, Qian ZZ, Fu K, Zhang HL, et al. (2012) Nimotuzumab combined with gemcitabine and cisplatin as second-line chemotherapy for advanced non-small-cell lung cancer. Thoracic Cancer 3: 72-78. Link: https://goo.gl/SDX0Li
- Mitchell P, Thatcher N, Socinski MA, Wasilewska-Tesluk E, Horwood K, et al. (2015) Tecemotide in unresectable stage III non-small-cell lung cancer in the phase III START study: updated overall survival and biomarker analyses. Ann Oncol 26: 1134-114. Link: https://goo.gl/kVxfIC
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
