Archives of Pulmonology and Respiratory Care
Hypoxia/Reoxygenation modulates Oxidative Stress Level and Antioxidative Potential in Lung Mitochondria: Possible participation of P53 and NF-KB Target Proteins
Department of Hypoxic States, Bogomoletz Institute of Physiology National Academy of Sciences of Ukraine, Ukraine
Author and article information
Cite this as
Gonchar O, Mankovska I (2017) Hypoxia/Reoxygenation modulates Oxidative Stress Level and Antioxidative Potential in Lung Mitochondria: Possible participation of P53 and NF-KB Target Proteins. Arch Pulmonol Respir Care. 2017; 3(1): 035-043. Available from: 10.17352/aprc.000022
Copyright License
© 2017 Gonchar O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background and objective: Hypoxia/reoxygenation (H/R) is a key factor in the pathogenesis of the most lung diseases where exсessive ROS production and prooxidant/antioxidant imbalance greatly contribute to disease progression. We have used severe hypoxia in sessions of repeated H/R of different duration as a model of lung pathologic states to investigate mitochondrial oxidative stress intensity, protein expression/activity of antioxidant enzymes manganese-superoxide dismutase (MnSOD), glutathione peroxidase (GPx), and antiapoptotic Bcl-2 as well as protein expression of their upstream regulators: p53 and nuclear factor- kappa B (NF-kB).
Methods: A total 86 rats were divided into five experimental groups and subjected to H/R [5 cycles of 10 min hypoxia (5.5 % O2 in N2) alternated with 10 min normoxia, daily]. Eight rats from each group were sacrificed on 1st -, 3rd - day, 1st and 2nd - week time points. Oxidative stress biomarkers (ROS formation, lipid peroxidation, H2O2 production, GSH/GSSG ratio, and mitochondrial aconitase activity as marker of compartment-specific superoxide anion production), indices of antioxidant status (MnSOD,GPx, glutathione –S-transpherase activities, and reduced glutathione level) were measured in lung mitochondria. Western blot was used to detect the protein levels of p53, Bcl-2, MnSOD, and GPx in mitochondria as well as the phosphorylated NF-kB p65 in the nucleus of lung cells. Expression of mRNA MnSOD was determined by real-time polymerase chain reaction.
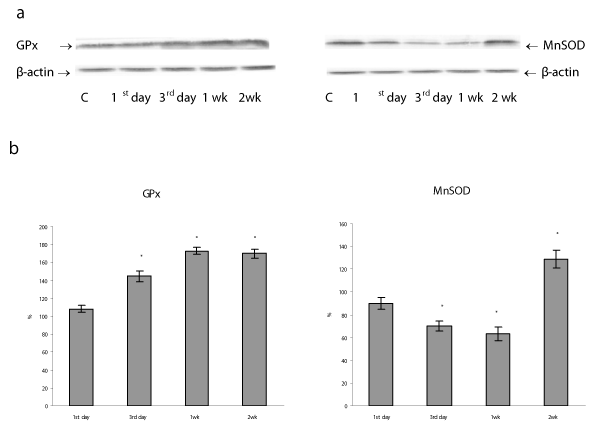
Results: The short- (1-3 days) and long-term (1-2 wk) H/R differentially affects the oxidative stress level, p53 protein expression and its subcellular distribution as well as antioxidant capacity in lung mitochondria.The long-term H/R caused mitochondrial p53 protein translocation, a decrease in Bcl-2 protein content, and a significant increase in nuclear accumulation of the phosphorylated NF κB p65 protein. We observed an increase in GPx protein content/activity, in parallel with decrease in MnSOD protein level and activity. In the dynamics of MnSOD gene expression we found a phase time point dependence.
Conclusions: Long lasting H/R leads to mitochondrial prooxidant/antioxidant disbalance that resulted in redox alteration as consequence of oxidative stress propagation and apoptotic cascade activation. A close correlation between mitochondrial p53 Protein level and protein expression/activities of its targets MnSOD and GPx suggest participation of p53 in regulation of H/R-induced mitochondrial oxidative stress level.
Introduction
The repeated episodes of hypoxia and reoxygenation (H/R) are wide spread phenomena because occurs in a variety of pathological conditions. Oxygen deprivation during hypoxia and subsequent reoxygenation are thought to be the major factors contributing to ROS production and oxidative stress development [1,2]. Indeed, there is increasing evidence that ROS generation and oxidant/antioxidant imbalance play a major role in a wide range of lung pathological processes including sleep disordered breathing, chronic obstructive pulmonary disease, asthma attacks, lung ischemia/reperfusion injury, etc [3-7]. Current findings suggest that the primary sensor of hypoxia is mitochondria, which increases the ROS production at low pressures of O2 and simultaneously is subject to direct attack by ROS. Lung mitochondria are protected against these oxidants by a variety of antioxidant mechanisms among which manganese- superoxide dismutase (MnSOD) and glutathione peroxidase (GPx) constitute not only the first line of defense but also are essential for maintaining the critical cellular redox balance and play a key role in modulating cellular responses to external stimuli [4, 6-8].
MnSOD is a homotetramer found exclusively in the mitochondrial matrix. Altered expression or enzyme activity of MnSOD has been linked to many pathologic conditions suggesting an important role for the modulation of mitochondrial ROS in the disease development [4,9,10]. The expression of MnSOD can be regulated at multiple levels from transcription and translation to posttranslational modifications by various extracellular and intracellular factors [4,11,12]. However, the precise mechanisms underlying MnSOD regulation in lung tissue at H/R of different duration are not clear.
The increase in the cell ROS often involves the activation of intracellular signaling pathways, which, in turn, regulates expression of a number of genes encoding antioxidant proteins, DNA repair proteins, stress-regulated chaperones, and antiapoptotic proteins [13]. The redox-sensitive transcription factors nuclear factor-kappaB (NF-kB) and p53 are considered as important stress sensors playing crucial role in determining cellular fate during oxidative stress [1,14,15]. Current data have showed that hyper-physiological and physiological levels of p53 exert different effects on cellular redox status either through directly regulating the expression of pro-oxidant and antioxidant genes or through modulating the cellular metabolic pathways [16,17]. P53 can upregulate the expression of various antioxidant enzymes such as aldehyde dehydrogenase 4, mammalian sestrin homologues that encode peroxiredoxins, and GPX1 [14]. The relationship between mitochondrial p53 protein level and both MnSOD and GPx were observed in many cell lines [16,17] supposing that p53 may regulate protein expression/activity of these antioxidant enzymes under oxygen deprivation.
NF-kB binding sites were found to be located in the regulatory regions of the SOD2 gene, which encodes MnSOD [18], providing direct evidence of the NF-kB – MnSOD connection. In addition, oxidative mechanisms may also regulate GPX1 gene transcription via NF-kB sites in the promoter region [19]. It is well known that the ROS-dependent NF-kB activation leads to an increase in MnSOD as well as GPx expressions [11,18,19].
Recent studies have shown that acute and long-term periodic hypoxia/reoxygenation differentially affect the various metabolic processes; in some cases, prolonged H/R was found to elicit a preconditioning-like effect. The duration, frequency, and severity of hypoxic episodes are critical factors determining whether exposure to cyclic H/R has beneficial or harmful effects on tissues [8]. Although oxidative damage associated with severe hypoxia/reoxygenation in lung tissue has been the subject of many studies [2-7], the knowledge of these effects in lung mitochondria, particularly concerning expression and activity of stress-inducible proteins, is relatively poor.
Based on previous research, the present study have been focused on the problem how the antioxidant defense system as well as p53 and NF-kB p65/RelA proteins produced by lung can be modulated by severe hypoxia in sessions of H/R of different duration. We assessed the mitochondrial oxidative stress level and redox status with an ample array of oxidative stress biomarkers and found that these changes were time- dependent, at once H/R influenced on the p53 and phosphorylated NF-kB p65 protein expression and distribution in cell compartments. The modulation of the protein expression/ activity of MnSOD, GPx, and ant apoptotic Bcl-2 as well as mRNA MnSOD expression in response to varying oxygen level were analyzed.
Materials and Methods
Animals and study design
Wistar rats weighing 220-260g were used. They were housed in Plexiglas cages (4 rats per cage) and maintained in an air-filtered and temperature controlled (20-22°C) room. Rats received a standard pellet diet and water ad libitum and were kept under artificial light-dark cycle of 12h. The present study was approved by the Animal Ethics Committee of Bogomoletz Institute of Physiology (Protocol No 15/37) and performed in accordance with the Guide for the Care and Use of Laboratory Animals. The rats were randomly divided into 5 groups. Group 1- control (C): rats were sedentary and under normoxic condition. Animals from the others groups were subjected to sessions of hypoxia/reoxygenation. Hypoxic episodes were created by breathing hypoxic gas mixture (5.5%O2 in N2) in normobaric condition in a special chamber. We used experimentally repeated short-term hypoxia (10 min) with normoxic intervals (10 min). Rats had such five sessions daily. Animals, which had sessions of hypoxia/reoxygenation were sacrificed after the 1st day (Group 2), 3rd day (Group 3), 1 week (Group 4) and 2nd weeks (Group 5) of experimental exposure (eight rats from each time point). Ambient O2 levels in the chamber were continuously monitored by use of a Beckman O2 analyzer (model OM-11) by sampling the air in the chamber. The duration of the gas flows during each hypoxic and normoxic episode was regulated by timed solenoid valves. Animals of each group were decapitated 24h after the last hypoxic session. At the time of sacrifice, the animals were lightly anaesthetized with ether.
Mitochondrial fraction preparation
Rat lung mitochondria were isolated by differential centrifugation. Tissue was collected in isolation medium A (250 mM sucrose, 10 mM Tris/ HCI (pH 7.6), 1mM EGTA and 0.5% defatted bovine serum albumin) and homogenized. After centrifugation of the homogenate at 1000g for 5 min, the supernatant was strained on gauze and recentrifuged at 12 000g for 15 min. The resulting pellet was resuspended in ice-cold isolation medium B (250 mM sucrose, 10 mM Tris/ HCI (pH 7.6) and 0.1 mM EGTA) and a new series centrifugation was performed. The final washing and resuspension of mitochondria was in the medium B without EGTA. Protein concentration was determined by the Bradford method, using bovine serum albumin as a standard.
Oxidative stress biomarkers assays
The data on ROS formation were obtained from dichlorofluorescein (DCF) fluorescence. Mitochondria were loaded for 20 min at 37°C with membrane permeable non-fluorescent probe (2’,7’-dichlorodihydrofluorescein diacetate, DCFHDA) which is known to be decomposed in the matrix to give dichlorofluorescein upon oxidation by matrix ROS, primarily hydroperoxide and superoxide. DCF was excited at 504 nm, and the emission was registered at 525 nm.
Lipid peroxidation in isolated mitochondria was measured from the formation of thiobarbituric acid - reactive substances (TBARS) using the method of Buege and Aust [20].
H2O2 concentration in lung mitochondria was measured by the FOX method, based on the peroxide-mediated oxidation of Fe2+, followed by the reaction of Fe3+ with xylenol orange [21]. The working reagent contained 500 μM ammonium ferrous sulfate, 50 mMH2SO4, 200 μM xylenol orange, and 200 mM sorbitol. Absorbance of the Fe3+-xylenol orange complex (A 560) was detected after 45 min. Hydrogen peroxide content was determined against calibration plot and calculated per 1 mg of mitochondrial protein.
Enzymatic assays
Activity of selenium-dependent glutathione peroxidase (GPx) was determined according to the method of Flohe and Gunzler [22]. Briefly, the reaction mixtures consisted of 50mM KPO4 (pH 7,0) 1mM EDTA, 1mM NaN3, 0,2 mM NADPH, 1 mM GSH, 0,25mM H2O2, 226 U/ml glutathione reductase, and rates of NADPH oxidation followed at 340 nm.
Glutathione-S-transferase (GST) activity was determined by assaying 1-chloro-2, 4-dinitrobenzene conjugation with GSH, as described by Warholm et al. [23].
Manganese superoxide dismutase (MnSOD) activity was measured by the method of Misra and Fridovich [24], which is based on the inhibition of autooxidation of adrenaline to adrenochrome by SOD contained in the examined samples. The samples were preincubated at OoC for 60 min with 6 mM KCN, which produces total inhibition of Cu, Zn-SOD activity. The results were expressed as specific activity of the enzyme in units per mg protein. One unit of SOD activity being defined as the amount of protein causing 50% inhibition the conversion rate of adrenaline to adrenochrome under specified conditions.
Aconitase activity was measured spectrophotometrically by monitoring the formation of cis-aconitate from isocitrate at 240 nm in 50 mM Tris HCl buffer (pH 7.4), containing 0.6 mM MnCl2 and 20 mM isocitrate at 250C[25].
Glutathione content assays
Total glutathione – the sum of reduced glutathione and oxidized glutathione (GSH and GSSG) − was determined by a method where glutathione is extracted from the lung mitochondria with 5% ice-cold-sulfosalicytic acid and after neutralization with triethanolamine sequentially oxidized by DTNB (0.6 mM) and reduced by NADPH (0.3 mM) in the presence of glutathione reductase (2 U/ml )[26]. For determination the GSSG alone, the GSH presented in solutions was derivatized by incubation with 2 µL 2-vinilpyridine at 40C for 1h. The rate of 2-nitro-5-thiobenzoic acid formation was monitoring at 412 nm and compared to a standard curves made with GSH and GSSG, respectively. The GSH concentration is calculated as total glutathione – 2 x [GSSG].
Western blot analysis
For immunoblotting analysis isolated mitochondria were incubated with RIPA buffer containing 50 mM Tris-HCI pH 8.0, 150 mM NaCI, 1.0% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulphate, 1mM phenylmethylsulfonyl fluoride, 1 µg/ml Halt TM Protease and Phosphatase inhibitor Coctail (78440, ThermoScientific Inc, USA). The lysate was centrifuged at 14,000g for 15 min. This fraction was labeled as the mitochondrial fraction and kept at -80o C. The cytosol fraction was performed as follows. The tissues was homogenized in ice-cold lysis buffer containing 10 mM HEPES (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 1mM dithiothreitol, 0.1mM EDTA, and 0.2mM phenylmethylsulfonyl fluoride (PMSF) plus 1 µg/ml Halt TM Protease and Phosphatase inhibitor Coctail (78440, ThermoScientific Inc, USA). This suspension was incubated on ice for 15 min. Then 12.5 µl of 10% Nonidet P-40 was added and the mixture was vigorously vortexed for 15s. The cytoplasmic and nuclear fractions were separated by centrifugation at 15,000g at 4 °C for 2 min.
Equal amounts of protein (100µg) was mixed with Laemmli buffer (S3401, Sigma), heated (99°C, 5 minutes), and then loaded onto 10-12% SDS polyacrylamide gels. Separated proteins were transferred onto polyvinylidene difluoride (PVDF) membranes which were blocked in 5% non-fat milk in Tris-buffered saline-Tween (TBS-T) for 1 hour at room temperature. Primary antibodies were applied overnight at 4°C. After washing in 1% non-fat milk in TBS-T (3 × 10 min), membranes were incubated with a secondary antibody conjugated to horseradish peroxidase (HRP) for 1 hour at room temperature. Each antigen-antibody complex was visualized by amino-ethylcarbazol reaction. The band intensities were quantified by densitometry with a computerized image processing system (GelPro Analyzer). Results were expressed as percentages of control values. β-Actin was used as a loading control. Antibodies and dilutions: p53 1:500 (Thermo Scientific Inc, USA); MnSOD 1:500 (Sigma-Aldrich Co); GPx 1/2 (B-6) 1:500 (Santa Cruz Biotechnology, Inc); Bcl-2 1:500 (Santa Cruz Biotechnology, Inc); phospho-NF-kBp65(Thermo Scientific Inc, USA); β-Actin 1:1000 (Santa Cruz Biotechnology, Inc); mouse anti-rabbit IgG HRP 1:1000 (Sigma-Aldrich Co).
RNA extraction and RT-PCR for MnSOD analysis
Total RNA was isolated using a commercial kit “Trizol RNA Prep100” (Isogen, Russia) according to the manufacturer’s instruction. Reverse transcription (RT) was performed using RevertAidTM H minus First Strand cDNA synthesis kit (Fermentas, Lithuania), 1.2-1.5 µg of total RNA and Random hexamer primers. Obtained one-strand cDNA was used for real-time PCR. PCR primers for Mn-SOD and glyceraldehyde-3-phosphatedhydrogenase (GAPDH) were purchased from “Fermentas” (Lithuania) and contained the following sequences: Mn-SOD sense primer: 5´ -CTGAGGAGAGCAGCGGTCGT-3´, MnSOD antisense primer: 5´- CTTGGCCAGCGCCTCGTGGT-3´; GAPDH sense primer: 5´ -GGGTGTGAACCACGAGAAAATATGA-3´, GAPDH antisense primer: 5´- AGCACCAGTGGATGCAGGGGATGAT-3´. Mixture for amplification contained 5µL of 5x PCR buffer, 1.5mM magnesium sulphate, 0.2mM of each dNTP, 3µl cDNA, 1U Tag-polymerase (AmpliSens, Russia), 30pM of each of the primers and deionized water to 25µL of total volume. The mixture was subjected to 40 cycles for Mn-SOD and 30 cycles for GAPDH of sequential steps in an automated thermal cycler “Applied Biosystems 2700” (Perkin Elmer, USA): denaturation (1 min at 94oC for Mn-SOD and GAPDH), annealing (40 s at 61 oC for Mn-SOD and 50 s at 65.5 oC for GAPDH), then elongation (1min at 72 oC for Mn-SOD and GAPDH). This program was complete with a final extension for 7 min at 72 oC. After amplification, the products were separated by electrophoresis on 1.6 % agarose gels and ethidium bromide-stained bands were visualized by UV transillumination (BioCom, Russia), the fluorescence intensity was quantified using a Gel Doc 2000 system (BioRad). There were no significant differences in intensity of GAPDH levels between experimental groups.
Statistical analysis
Data are expressed as mean ± standard deviation for each group. The differences among multiple experimental groups were detected by one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test. The correlation between pairs of variables was analysed using the bivariate Pearson method. A P value of less than 0.05 was considered as significant.
Results and Discussion
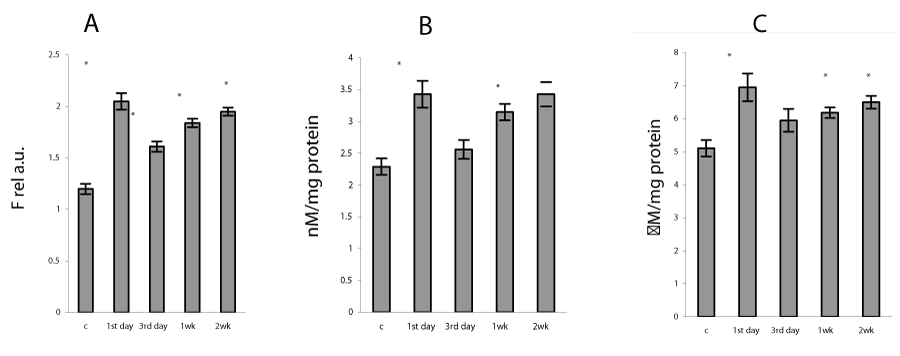
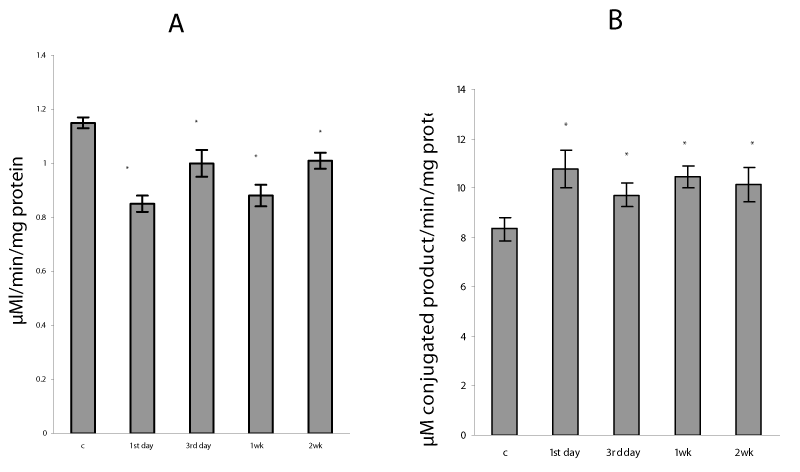
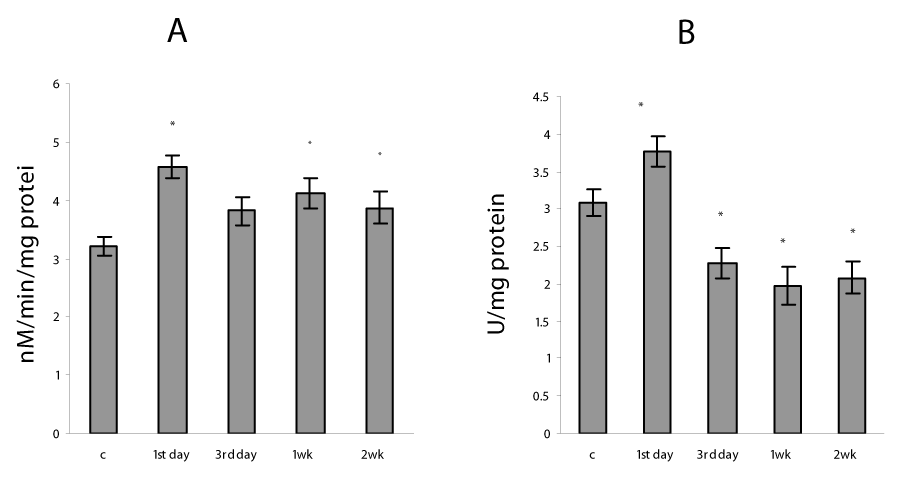
In the present study, the severe hypoxia using in sessions of intermittent hypoxia caused in lung mitochondria a significant increase in ROS formation as well as a rise in TBARS and H2O2 production (Figure 1). It is known that hypoxia as well as reoxygenation can induce excessive ROS generation as a result of the univalent reduction of molecular oxygen to O2 ∙− by electrons that leak from the mitochondrial electron transport chain, mainly from complexes I and III [1]. Our data confirmed that mitochondrial oxidative stress was involved already at an early phase of H/R condition - on the first day of exposure and continued the whole study period for 2 weeks. The intensity of the oxidative processes reduced on 3rd day and gradually enhanced at 1 and 2 weeks of H/R exposure (Figure 2). These data are in agreement with the results described in other model systems, which demonstrated a significant increase in lipid peroxidation measured in lung homogenates [2,3,7] and mitochondria [27]. The intensification of oxidative processes in mitochondria was accompanied by an increase in GSSG content and decrease in GSH concentration as well as in GSH/GSSG ratio (P<0.05) (Table 1), which are essential indicators of oxidative stress and mitochondrial dysfunction [6,8]. In addition, we registered a decrease in mitochondrial aconitase activity (Figure 2), which evidences an increase in superoxide anion production in mitochondria. Moreover, inactivation of aconitase may block normal electron flow to oxygen, leading to an accumulation of reduced metabolites such as NADH. This condition has been termed ‘‘reductive stress,’’ and can causes an increased production of reactive oxygen species through autoxidation of the reduced metabolites, thus further increasing oxidative damage [28].
Generally, an increased production of ROS triggers an array of signal transduction pathways, resulting in stimulatory or inhibitory output signals [13]. In the present study, we have investigated whether sessions of repeated H/R alters lung mitochondrial antioxidant capacity, regarding both protein expression and activity, and whether these alterations could be related to their transcriptional regulators p53 and NF-kB. To determine the mechanisms of H/R influence on the oxidative stress level as well as redox balance, we measured p53, NF-kB, MnSOD, and GPx protein expression in cellular compartments by Western blot analyses and mRNA MnSOD by RT PCR.
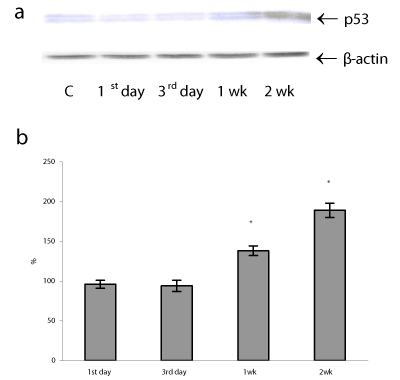
In lung mitochondria, simultaneously with a rise of oxidative state, we registered changes in the protein content of p53 in both cell compartments (cytosol and mitochondria) and these changes were time-dependent. The data (not shown) suggest that the disappearance of p53 protein from cytoplasm at long-lasting period of H/R resulted in p53 protein translocation to mitochondria and perhaps to the nucleus as an active form, because, it is seems that p53 translocation to mitochondria precedes its nuclear translocation [29]. Thus, in lung mitochondria the p53 protein content was significantly elevated after prolonged H/R exposure by 38 and 89 % (P<0.05) on 1 and 2 weeks of H/R, while at an early period of H/R (1-3 days) p53 protein levels remained close to the basal level (Figure 3). It should be noted that in the late phase of our study the severity of mitochondrial oxidative stress (ROS formation) positively correlated with the mitochondrial p53 protein content (r=0.87), indicative of that p53 cell distribution may be ROS-regulated.
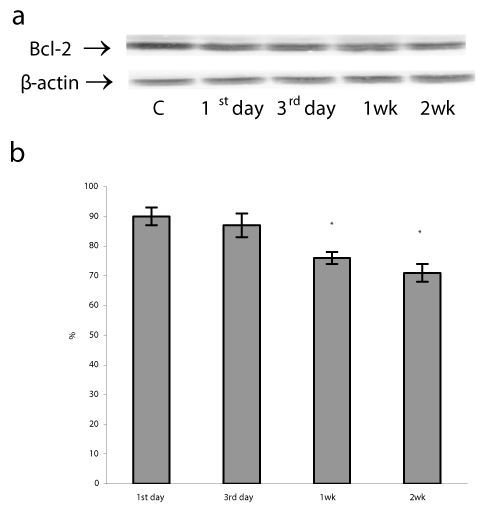
A mitochondrial translocation of p53 was observed in a variety of human and mouse cells under various types of DNA damage, hypoxia, oncogene deregulation, and oxidative damage [14,16,29]. P53 protein accumulation in mitochondria can induce transcription - independent apoptosis through direct interaction p53 with Bcl-2 family proteins which are located in the outer membrane of mitochondria [14,30]. Anti-apoptotic Bcl-2 proteins serve as a known sensor of apoptotic signaling that prevent apoptosis and are an important factor determining the fate of a DNA-damaged cell [31]. In addition, in mitochondria p53 induces pro-apoptotic Bax and Bak oligomerization, which antagonizes the Bcl-2 and Bcl-xl antiapoptotic effect, and forms a complex with cyclophilin D in the mitochondrial inner membrane. These changes result in the marked disruption of mitochondrial membranes and subsequent cytochrome c release and procaspase-3 activation [32]. In parallel with p53 mitochondrial translocation, we found a decrease in Bcl-2 protein levels after the 3rd day and then during 1st and 2nd weeks of H/R exposure by 10, 24, and 29%, respectively (P<0.05) (Figure 4). These findings demonstrate that the prolonged H/R can induce apoptotic cascade activation by decreasing of Bcl-2 protein content and the duration of H/R has a direct effect on the viability of lung cells.
It is known that the relationship between p53 and ROS is quite complex. On the one hand, excessive ROS may cause p53 translocation to mitochondria and enhance mitochondrial oxidative stress leading to apoptosis. On the other hand, p53 can also affect ROS production and pro-/antioxidative balance in mitochondria by impact on its targets MnSOD and GPx [13,16,17].
We found that the repetitive situation of severe hypoxia followed by reoxygenation caused a disturbance of the mitochondrial pro-oxidant/antioxidant homeostasis, which manifestated in the alteration of MnSOD and GPx protein expressions/activities. Thus, in lung mitochondria there was observed the increase in GPx protein content from 3rd day to 1 and 2 weeks of H/R by 45, 73 and 60%, respectively (P<0.05) (Figure 5), GPx activity was enhanced during the whole study period (Figure 6). The MnSOD protein content as well as mRNA MnSOD expression did not significant change from control level after the first day of H/R, but the MnSOD protein content decreased from 3rd day to 1st week and increased by 29% (P<0.05) after 2nd week of H/R exposure (Figure 5). In the dynamics of MnSOD gene changes we found a phase dependence, namely a decrease on the third day and an increase after the first and second weeks of H/R exposure (Figure 7). At the same time, the MnSOD activity diminished during the whole study period by 30, 26, 36, 32 %, P<0.05 (Figure 6). These evidences indicate that mitochondrial superoxide radical is a potentially critical effector in severe H/R-induced oxidative processes and may explain the decrease in mitochondrial aconitase activity (Figure 2), because O2•− is the main factor responsible for the disruption of the [4Fe-4S]2+ cluster present in the enzyme and the abolition of aconitase functional activity [28].
In our study, mitochondrial p53 protein content negatively correlated with MnSOD activity (r = - 0.62) and positively with the MnSOD (r=0.59) and GPx (r =0.66) protein content. Our observations suggest that under H/R condition p53 protein level actively affect the MnSOD specific activity in lung mitochondria. As previously reported by others, p53 not only inhibits MnSOD superoxide scavenging activity by physically interacting with the enzyme in mitochondria, but also is linked to the regulation of MnSOD protein levels since p53 shows a dual role: at low concentration p53 increases MnSOD protein levels, whereas at high concentration p53 decreases MnSOD expression [16,17,29]. However, the actual mechanism of MnSOD regulation can be more complicated with other players possibly being involved. Thus, Pani et al. have recently proposed that high proapoptotic levels of p53 induce p66shc, thus favoring H2O2 generation at the mitochondrial electron transport chane; peroxide may diffuse to cytosol where it triggers the redox-dependent inhibition of FOXO3a, leading to reduced expression of ROS scavengers including MnSOD, and to further oxidative stress. In parallel, p53 reduces MnSOD gene transcription by inhibiting of SP-1, but acts also on MnSOD protein by binding to the enzyme in mitochondria. This pro-oxidant network is counteracted by Sirt-1 that increases MnSOD levels in two ways: by deacetylating and inhibiting of p53, and by directly activating of FOXO3a. In addition, severe hypoxia in sessions of H/R can increase mitochondrial peroxynitrite formation that ultimately lead to nitration and inactivation of MnSOD [33].
Our experimental data showed the decrease in content of the reduced glutathione, which are in agreement with several early reports that severe hypoxia depletes GSH stores in lung mitochondria as well as in lung homogenates [2,27] and leads to a short-term fall in intracellular GSH levels in rat endothelial and alveolar cells both in vitro and in vivo [3,6]. Depletion of the reduced glutathione seems to be occurred by conjugation reactions via the GST, which activity in this study has arisen (Figure 2). GSTs are a family of dimeric proteins actually regarded as a major detoxification tool against oxidative damage, as they catalyze the conjugation to GSH of a wide number of toxic elecrophilic compounds produced under oxidative stress conditions [5,6,8]. Moreover, changes in the GSH/GSSG ratio, which we found under H/R condition (Table 1) can regulate H2O2 formation, alter protein redox status and in that way modulate many signaling pathways including the activation of NF-kB [34-35].
Previously, we have demonstrated that acute severe hypoxia induces destructive changes within lung parenchyma, including the surfactant system-forming structures (delamellation of type II pneumocytes, edematous changes, damage to the alveolar lining layer, and accumulation of alveolar macrophages) [2]. In addition to cellular membrane damage and alteration in the lung lipid composition, the oxygen deprivation produced lung inflammation due to neutrophil influx into the airways, which was associated with NF-kB activation and the high expression of IL-8 mRNA in alveolar macrophages and, to a lesser extent, in alveolar epithelial cells [1,7]. Emerging data indicate that chronic intermittent hypoxia as well as high altitude exposure cause NF-κB activation and induce production of proinflammatory chemokines, cytokines, and adhesion molecules contributing to tissue injury, which, in turn, activates signaling cascades to enhance NF-κB activation [1, 3,36].
NF-kB is a ROS regulated and redox-sensitive factor that normally is confined to the cytoplasm in unstimulated cells and translocates to the nucleus in response to various stimuli including oxidative stress [13]. It is known, that under resting conditions, NF-κB is bound to its co-repressor molecule IκB in the cytosol. Upon stimulation, like intermittent hypoxia, IκB is degraded and allows p50 and p65 to form NF-κB heterodimer, which then translocates to the nucleus and activates transcription of target genes, such as inflammatory factors and prosurvival proteins [15]. The NF-kB activation is provided by a series of redox-sensitive protein kinases that promote the dissociation of the IkB–NF-kB complex by phosphorylation of either IkB or NF-kB. It was also observed that a decrease in the intracellular pool of GSH followed by a subsequent increase in the level of GSSG, as well as a rise in H2O2 formation mediate IkBa phosphorylation and subsequent activation of NF-kB [34,35,37].
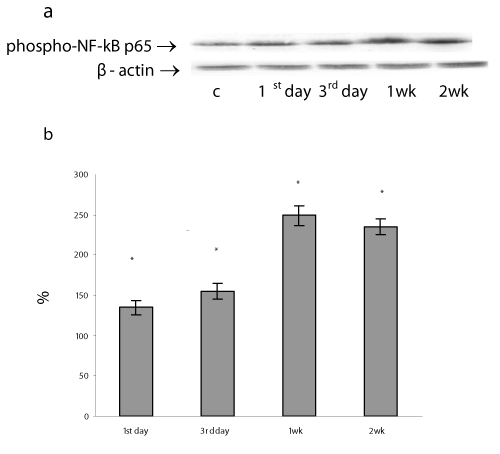
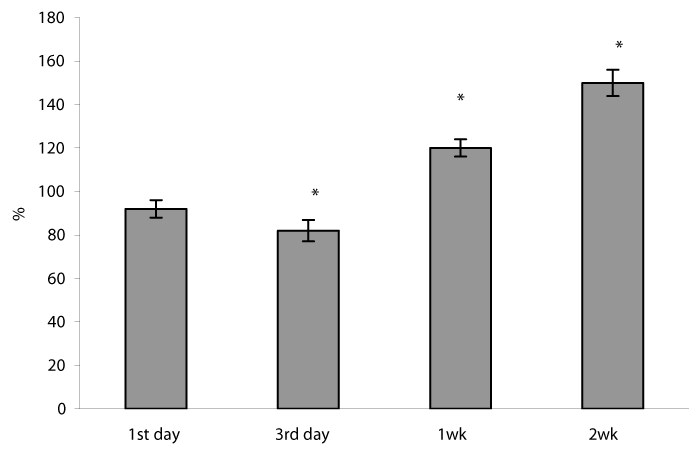
The phosphorylation of the subunit p65/RelA displays the NF-kB activation and is required for expression of a subset of NF-kB – dependent genes [38]. We measured the amount of phosphorylated NF-kB p65 by Western blot in nuclear extracts of lung cells sampled at different time points following sessions of H/R. Our results have shown the nuclear accumulation of the phosphorylated NF κB p65 protein in lung cells gradually increased during the whole study period by 35, 55, 149, 135% on 1st , 3rd , 1st and 2nd weeks of H/R exposure, respectively, P<0.05) (Figure 8). Theoretically, the activated NF κB p65 can be translocated to the nucleus to promote the transcription of the target genes including pro-survival MnSOD, GPx, and Bcl-2 [35]. In our experimental model the phosphorylated NF-kB p65 protein accumulation positively correlated with the ROS formation (r = 0.58) and GPx protein content (r=0.96). These findings are consistent with previous studies showing that the high level of oxidative and nitrosative stresses promoted the induction of GPx mRNA transcription as well as protein expression in various cell lines [39] including via NF-kB sites in the promoter region [19]. Furthermore, a close correlation was identified between phosphorylated NF-kB p65 protein content and mRNA MnSOD expression (r =0.74). All these observations as well as an increase in MnSOD protein content after 2 weeks of H/R exposure make it possible to assume that long-lasting H/R can induce MnSOD protein and mRNA expression via NF-B activation. Early, it was demonstrated that hypoxia decreased the both MnSOD activity and protein expression in rabbit lung [40] and decreased MnSOD mRNA expression in cultured rabbit alveolar type II epithelial cells and lung fibroblasts after exposed to 5% hypoxia [41]. On the other hand, some authors demonstrated an increase in the MnSOD protein expression and MnSOD mRNA content in rat lungs [42]. In our previous research, we found that prolonged moderate hypoxia/hyperoxia enhanced MnSOD protein expression without changes in mRNA MnSOD content in lung tissue [27].
It is widely known that MnSOD regulation is complex and occurs at both pre- and post-translational levels. It was reported that MnSOD mRNA levels can be up-or downregulated by several factors including VEGF, AP-2, Egr-1, Sp-1, p53, HIF-2 alpha, PKC-NF-κB, PI3K-Akt-Forkhead signaling pathways [1,11-14,18,33]. These facts indicate that the overall regulation of Mn-SOD is multifactorial and cross-talk between redox-sensitive pathways are required for synergic activation of MnSOD gene. Which of these signaling pathways is involved in the regulation of MnSOD at H/R condition is apparently dependent on the strength, duration, and type of hypoxic exposure. We consider that this problem requires attention for further researches.
Conclusion
We found that short- and long-term H/R differentially effects on p53 cellular distribution, mitochondrial oxidative stress level and antioxidant capacity. Prolonged H/R as opposed to short-term caused p53 mitochondrial accumulation, mitochondrial prooxidant/antioxidant disbalance, a decrease in Bcl-2 protein content, a significant increase in nuclear accumulation of the phosphorylated NF κB p65 protein. A close correlation between mitochondrial p53 protein level and protein expression/activities of its targets MnSOD and GPx suggest participation of p53 in regulation of H/R-induced mitochondrial oxidative stress. Our findings that MnSOD transcription and expression as well as NF-kB activity were higher than control level after 2 weeks of H/R do not exclude the assumption of the beginning of the adaptative process formation in lung cells to oxygen fluctuations.
According to currently available literature, our study provides additional information, which may help to understand molecular mechanisms of conversion of short-term and prolonged H/R- induced oxidative stress into mitochondrial dysfunctions and to find the effective therapeutic interventions to control oxidative stress in the lung tissue for preventing of oxidative injury.
We would like to thank N. Petrova and N. Steshenko for technical assistance.
- Li C, Jackson RM (2002) Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol 282: 227-241. Link: https://goo.gl/YcIYsc
- Gonchar O, Rozova K (2007) Effects of different modes of interval hypoxic training on morphological characteristics and antioxidant status of heart and lung tissues. Bull Exp Biol Med 144: 249-252. Link: https://goo.gl/hmx7Mk
- Araneda O, Tuesta M (2012) Lung oxidative damage by hypoxia. Oxid Med Cell Longev. Link: https://goo.gl/9iPnuP
- Kinnula VL, Crapo JD (2003) Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med 167: 1600-1619. Link: https://goo.gl/5xW9B7
- Comhair SA, Erzurum SC (2002) Antioxidant responses to oxidant-mediated lung diseases. Am J Physiol Lung Cell Mol Physiol 283: 246-55. Link: https://goo.gl/tpNdRH
- Rahman I, Biswas SK, Kode A (2006) Oxidant and antioxidant balance in the airways and airway diseases. European Journal of Pharmacology 533: 222-239. Link: https://goo.gl/C5m5Cy
- Minko T, Stefanov A, Pozharov V (2002) Lung hypoxia: antioxidant and antiapoptotic effects of liposomal ?-tocopherol. J Appl Physiol 93:1550-1560. Link: https://goo.gl/s7H5V6
- Gonchar O, Mankovskaya I (2010) Antioxidant system in adaptation to intermittent hypoxia. J Biol Sci 10:545-554. Link: https://goo.gl/FzeeKj
- Holley AK, Bakthavatchalu V, Velez-Roman JM, Clair DK (2011) Manganese Superoxide Dismutase: Guardian of the Powerhouse. Int J Mol Sci 1: 7114-7162. Link: https://goo.gl/syTkxl
- Pardo M, Tirosh O (2009) Protective signalling effect of manganese superoxide dismutase in hypoxia-reoxygenation of hepatocytes. Free Radic Res 43: 1225-1239. Link: https://goo.gl/CCTP6G
- Miao L, Clair DK (2009) Regulation of superoxide dismutase genes: Implications in disease. Free Radic Biol Med 47: 344-356. Link: https://goo.gl/UW1MfS
- Yamakura F, Kawasaki H (2010) Post-translational modifications of superoxide dismutase. Biochim Biophys Acta 1804: 318-325. Link: https://goo.gl/L23aow
- Ma Q (2010) Transcriptional responses to oxidative stress: Pathological and toxicological implications. Pharmacol Ther 125:376-393. Link: https://goo.gl/fwxuj6
- Liu B, Chen Y, Clair D (2008) ROS and p53: a versatile partnership. Free Radic Biol Med 44: 1529-1535. Link: https://goo.gl/muO12U
- Nakajima S, Kitamura M (2013) Bidirectional regulation of NF-?B by reactive oxygen species: A role of unfolded protein response. Free Radic Biol Med 65:162-174. Link: https://goo.gl/w8Tk5I
- Drane P, Bravard A, Bouvard V, May E (2001) Reciprocal down-regulation of p53 and SOD2 gene expression implication in p53 mediated apoptosis. Oncogene 20. Link: https://goo.gl/qYuoFM
- Pani G, Bedogni B, Anzevino R, Colavitti R, Palazzotti B, et al. (2000) Deregulated manganese superoxide dismutase expression and resistance to oxidative injury in p53-deficient cells. Cancer Research 60: 4654-4660. Link: https://goo.gl/VO3mMj
- Dhar S, Xu Y, Clair D (2010) Nuclear factor ?B- and specificity protein 1-dependent p53-mediated bi-directional regulation of the human manganese superoxide dismutase gene. J Biol Chem 285: 9835-9846. Link: https://goo.gl/OlLNKX
- Zhou L, Johnson A, Rando T (2001) NF- kappa B and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radic Biol Med 31:1405-1416 PMID:11728812. Link: https://goo.gl/jOeia1
- Buege J, Aust S (1978) Microsomal lipid peroxidation. Methods Enzymol LII: 302-308. Link: https://goo.gl/tTnkk1
- Wolff S (1994) Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol 233:182-189. Link: https://goo.gl/H6O2dg
- Flohe L, Gunzler WA (1984) Assay of glutathione peroxidase. Methods Enzymol 105: 114-120. Link: https://goo.gl/6Eos9D
- Warholm M, Guthenberg C, Bahr C, Mannervik B (1985) Glutathione transferases from human liver. Methods Enzymol 113: 499-501. Link: https://goo.gl/KD5w9T
- Misra H, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay superoxide dismutase. J Biol Chem 247: 3170-3175. Link: https://goo.gl/kOVvKx
- Hausladen A, Fridovich I (1996) Measuring nitric oxide and superoxide: rate constants for aconitase reactivity. Methods Enzymol 269: 37-41. Link: https://goo.gl/qgguhI
- Anderson M (1985) Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol 113: 548-551. Link: https://goo.gl/ooNTng
- Gonchar O, Mankovska I (2012) Moderate hypoxia/hyperoxia attenuates acute hypoxia-induced oxidative damage and improves antioxidant defense in lung mitochondria. Acta physiol Hung 99: 436-446. Link: https://goo.gl/wezjgd
- Bulteau A, Ikeda-Saito M, Szweda L (2003) Redox-dependent modulation of aconitase activity in intact mitochondria. Biochemistry 42:14846-14855. Link: https://goo.gl/iZLYBJ
- Zhao Y, Chaiswing L, Velez JM, Batinic-Haberle I, Colburn NH, et al. (2005) p53 translocation to mitochondria precedes its nuclear translocation and targets mitochondrial oxidative defense protein-manganese superoxide dismutase. Cancer Research 65: 3745-3750. Link: https://goo.gl/eLbwql
- Koumenis C, Alarcon R, Hammond E, Sutphin P, Hoffman W, et al. (2001) Regulation of p53 by hypoxia: Dissociation of transcriptional repression and apoptosis from p53-dependent transactivation. Mol Cell Biol 21:1297-1310. Link: https://goo.gl/nn8UEi
- Speidel D (2010) Transcription-independent p53 apoptosis: an alternative route to death. Trends in Cell Biology 20: 14-24. Link: https://goo.gl/mf12bP
- Dong JW, Zhu HF, Zhu WZ, Ding HL, Ma TM, et al. (2003) Intermittent hypoxia attenuates ischemia/reperfusion induced apoptosis in cardiac myocytes via regulating Bcl-2/Bax expression. Cell Research 13: 385-391. Link: https://goo.gl/sqmU6a
- Pani G, Galeotti T (2011) Role of MnSOD and p66shc in Mitochondrial Response to p53. Antioxid Redox Signal 15: 1715-1727. Link: https://goo.gl/AIyhC6
- Oliveira-Marques V, Marinho HS, Cyrne L, Antunes F (2009) Role of hydrogen peroxide in NF-kB activation: from inducer to modulator. Antioxid Redox Signal 11: 2223-2243. Link: https://goo.gl/sKrEyP
- Morgan MJ, Liu Z (2011) Crosstalk of reactive oxygen species and NF-?B signaling. Cell Research 21:103-115. Link: https://goo.gl/HNZZ0Y
- Sarada S, Himadri P, Mishra C, Geetali P, Ram MS, et al. (2008) Role of oxidative tress and NFkB in hypoxia-induced pulmonary edema. Exp Biol Med 233:1088-1098. Link: https://goo.gl/xu1m9d
- Kabe Y, Ando K, Hirao S, Yoshida M, Handa H (2005) Redox regulation of NF-kB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal 7: 395-403. Link: https://goo.gl/xvPnbv
- Gloire G, Piette J (2009) Redox regulation of nuclear post-translational modifications during NF-kB activation. Antioxid Redox Signal 11. Link: https://goo.gl/Y915PK
- Arai M, Imai H, Koumura T, Yoshida M, Emoto K, et al. (1999) Mitochondrial phospholipids hydroperoxide glutathione peroxidase plays a major role in preventing oxidative injury to cells. J Biol Chem 274 : 4924-4933. Link: https://goo.gl/9A5SgH
- Russell WJ, Ho YS, Parish G, Jackson RM. (1995) Effects of hypoxia on MnSOD expression in mouse lung. Am J Physiol 269: L221-L226. Link: https://goo.gl/1jiDUz
- Jackson RM, Parish G, Ho Y (1996) Effects of hypoxia on expression of superoxide dismutase in cultured ATII cells and lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 271: L955-L962. Link: https://goo.gl/sujwKs
- Shen C, Lee J, Su C, Wang D, Chen C (2008) Hypoxia and reoxygenation of the lung tissues induced mRNA expressions of superoxide dismutase and catalase and interventions from different antioxidants. Transplant Proc 40:2182-2184.s Link: https://goo.gl/Y6pGMQ
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
