Archives of Pulmonology and Respiratory Care
Clinical features and the prognostic significance of ocular sarcoidosis
1Department of Internal Medicine Medicine, Cerrahpasa Medical Faculty, Istanbul University, Turkey
2Department of Pulmonary Medicine, Cerrahpasa Medical Faculty, Istanbul University, Turkey
3Department of Opthalmology, Cerrahpasa Medical Faculty, Istanbul University, Turkey
Author and article information
Cite this as
Yanardag H, Tetikkurt C, Bilir M, Pazarlı H (2018) Clinical features and the prognostic significance of ocular sarcoidosis. Arch Pulmonol Respir Care. 2018; 4(1): 006-011. Available from: 10.17352/aprc.000032
Copyright License
© 2018 Yanardag H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The main objective of this study was to evaluate the clinical features of ocular sarcoidosis. The second aim was to assess the influence of eye involvement on the prognostic outcome, extrapulmonary organ, and endobronchial involvement in sarcoidosis patients. Third objective was to determine the contribution of ocular involvement to final diagnosis in patients presenting with an equivocal sarcoidosis diagnosis due to single organ disease.
One hundred seventy two sarcoidosis patients paticipated in the study and were classified into two groups according to the existence of eye involvement. The patients were evaluated retrospectively in regard to clinical features, prognostic outcome, extensity of extrapulmonary organ, and endobronchial involvement between the two groups. Eighty patients (46.5%) had eye sarcoidosis. Ocular involvement was the initial symptom in 15 subjects (18.7%) while asymptomatic ocular sarcoidosis was observed in 14 patients (17.5%) at initial admission. Red eye was the most common symptom occuring in 26 patients (32.8%) while anterior uveitis was the most frequent site (38.4%) of involvement. The other relevant ocular structures in the order of decreasing frequency were posterior uvea, papilla, lacrimal gland, and sclera. Patients with ocular sarcoidosis had a worse prognosis and a more extensive extrapulmonary organ involvement than the patients without eye disease. Although endobronchial involvement was more common in these patients, it was not statistically significant. In 12 patients (15%) with an equivocal sarcoidosis, identification of eye involvement confirmed the final diagnosis.
The results of our study reveal that the identification of ocular sarcoidosis appears to be a useful decisive clinical marker for determining the prognostic outcome of sarcoidosis patients. The incidence of extrapulmonary organ involvement was significantly higher in patients with ocular sarcoidosis. Detection of ocular disease was also useful for the final diagnosis in patients with a single organ sarcoidosis manifesting an indecisive clinical presentation. Recognition of ocular sarcoidosis is a rewarding and a preliminary clinical utility in sarcoidosis both for diagnosis and disease outcome while this finding may be an evidence of endobronchial involvement.
Introduction
Ocular involvement of sarcoidosis is known for a long time and has become more outstanding in the recent years. Silent clinical profile and various ocular compartment involvement has lead to a challenging portrait for the diagnosis of eye sarcoidosis [1,2]. The incidence of ocular sarcoidosis varies from 13% to 79% of the patients with systemic disease while ocular involvement is the presenting symptom in approximately one third. In patients with uveitis, 4% to 6% may develop clinical sarcoidosis [3-6], later in the course of disease.
Ocular sarcoidosis imitates many other primary ocular diseases because the disease can involve any structure in or around the eye. Eye involvement may be the first manifestation of sarcoidosis without any other organ involvement in approximately 20% to 30% of the patients [4,7]. The high prevalance of eye sarcoidosis reaching up to 79% of patients with systemic sarcoidosis [3] and the lack of physical or laboratory findings relevant for eye involvement [7] causes a diagnostic dilemma for the clinician especially in the patient without a previous history of sarcoidosis. Visual prognosis of ocular sarcoidosis may vary depending on the severity and chronicity of eye inflammation [8,9], that makes the early identification crucial for treatment.
The aim of our study was to investigate the clinical and prognostic features of ocular sarcoidosis. Another objective was to evaluate the influence of ocular involvement on extrapulmonary organ and endobronchial disease in sarcoidosis patients. The third intention was to evaluate the diagnostic utility of ocular sarcoidosis for the final diagnosis in equivocal cases presenting only with single organ disease.
Materials and Methods
This is a retrospective cohort study including 172 sarcoidosis patients who were evaluated between January 1988 and April 2018 at the Internal Medicine Department of Cerrahpasa Medical Faculty. The study has been approved by the IRB/Ethics Committee of Cerrahpasa Medical Faculty (01/03/2016-82556). Patients fulfilled the American Thoracic Society/European Respiratory Society criteria of sarcoidosis [10,11]. All subjects underwent pulmonary function tests, DLCO/VA [Carbon monoxide diffusion capacity of the lung corrected for alveolar volume (DLCO/VA)], chest x-ray, abdominal ultrasound, FOB [Fiberoptic Bronchoscopy (FOB)], thorax CT [Computed Tomography (CT)], slit lamp biomicroscopy, and fundus examination. Conjuntival, lacrimal gland, or other ocular tissue biopsies were performed from the suspected lesions. Ocular sarcoidosis was diagnosed by opthalmologic examination and the presence of noncasefied granulomatous inflammation pattern identified by the histopathologic examination of the ocular biopsy samples. The international criteria for the diagnosis ocular sarcoidosis were used to identify presence of eye involvement [9]: granulomatous keratic precipitates, iris nodules, trabecular meshwork nodules, vitreous opacities, multiple chorioretinal peripheral lesions, nodular or segmental phlebitis, retinal macroaneurysm in an inflamed eye, optic disc nodules, solitary choroidal nodule, and bilateral involvement. Conjunctival biopsy was performed in the presence of nodules or follicles in twelve and randomly in eight patients without visible lesions while lacrimal gland was biopsied in eight patients.
Laboratory investigations included complete blood count, liver function, renal function tests, serum Ca, 24h urinary Ca, erythrocyte sedimentation rate, C-reactive protein and ACE [Angiotensin-Converting Enzyme (ACE)]. Abnormal liver or renal function tests, high serum ACE, hypercalcemia and hypercalcuria were determined to be present if they were above the normal laboratory range. The DeRemee criteria; stage 0: normal, stage 1: bilateral hilar lymphadenopathy, stage 2: bilateral hilar lymphadenopathy and parenchymal involvement, stage 3: parenchymal involvement only, and stage 4: pulmonary fibrosis [12] were used for stage evaluation. Pulmonary function tests and DLCO/VA were performed according to the ATS/ERS criteria and the results interpreted in accordance with the ATS guidelines [13]. DLCO/VA was measured with the single-breath technique that was adjusted for alveolar ventilation. Values for the pulmonary function tests and DLCO/VA were evaluated as abnormal if they were outside the 95% confidence interval of the predicted values. Restrictive disease was revealed by a reduced TLC [Total Lung Capacity (TLC)] or FVC [Forced Vital Capacity (FVC)] and a normal or a high FEV1/FVC [Forrced Expiratory Volume in one second to Forced Vital Capacity ratio (FEV1/FVC)]. Diffusion capacity indicated by a DLCO/VA< %80 value was denoted as abnormal. All patients were screened by a dermatologist, neurologist, and an opthalmologist for the evaluation systemic involvement. Involvement of central nervous system was determined to exist if neurologic examination was positive, a lesion was identified by CT [Computed Tomography (CT)] or MRI (Magnetic Resonance Imaging (MRI)], and identified by a consultant neurologist.
Epidemiological, clinical, and histopathologic findings were obtained from the medical records of the patients. Bronchoscopy was performed under local anesthesia. Six bronchial biopsies were taken from each patient with an abnormal mucosa. In patients with a normal appearing mucosa eight biopsies from different sites and main carenas of both lungs were taken. The patients were evaluated as AOS [Absence of Ocular Sarcoidosis (AOS)] and POS [Presence of Ocular Sarcoidosis (POS)] according to the existence of ocular involvement. Extrapulmonary organ involvement was classified into two groups as LOI [Limited Extrapulmonary Organ Involvement (LOI)] if less than three organs were involved and as EEI [Extensive Extrapulmonary Organ Involvement (EOI)] when three or more organs were comprised. Endobronchial disease was denoted as LEI [Limited Endobronchial Involvement (LEI)] in the presence of one positive bronchial biopsy sample and as DEI [Diffuse Endobronchial Involvement (DEI)] if two or more samples were positive for noncasefied granulomatous inflammation. Sarcoidosis activity outcome were evaluated in regard to progressive stage, detoriation of pulmonary function tests, permanent decline of DLCO/VA values, extrapulmonary organ involvement, and the presence of severe systemic symptoms.
Epidemiological, clinical, and histopathologic findings were obtained from the medical records of the patients. Bronchoscopy was performed under local anesthesia. Six bronchial biopsies were taken from each patient with an abnormal mucosa. In patients with a normal appearing mucosa eight biopsies from different sites and main carenas of both lungs were taken. The patients were evaluated as AOS [Absence of Ocular Sarcoidosis (AOS)] and POS [Presence of Ocular Sarcoidosis (POS)] according to the existence of ocular involvement. Extrapulmonary organ involvement was classified into two groups as LOI [Limited Extrapulmonary Organ Involvement (LOI)] if less than three organs were involved and as EEI [Extensive Extrapulmonary Organ Involvement (EOI)] when three or more organs were comprised. Endobronchial disease was denoted as LEI [Limited Endobronchial Involvement (LEI)] in the presence of one positive bronchial biopsy sample and as DEI [Diffuse Endobronchial Involvement (DEI)] if two or more samples were positive for noncasefied granulomatous inflammation. Sarcoidosis activity outcome were evaluated in regard to progressive stage, detoriation of pulmonary function tests, permanent decline of DLCO/VA values, extrapulmonary organ involvement, and the presence of severe systemic symptoms.
One hundred twenty patients were treated with corticosteroids, thirty eight patients received azathioprine, and eighteen patients were commenced on methotrexate. Laboratory investigation during the follow-up period included blood count, serum biochemistry, serum and 24h urinary calcium. Lung function tests FEV1, FVC, TLC, and DLCO/VA were done every six months while ABG analysis was performed when indicated. The outpatient control was scheduled every three to six months according to the clinical profile of the patient. The mean follow-up period was 106.4±24.8 months. Chronic persistent disease was identified if severe pulmonary, systemic or extrapulmonary organ involvement manifestations arose, if severe pulmonary function impairment or significant deterioration of radiologic findings were observed. It is well-known that sarcoidosis often resolves within two to five years after initial diagnosis [14-17] while patients with incessant disease five years following diagnosis are classified as having chronic persistent disease. Active inflammation refractory to treatment that persisted more than two years from diagnosis decreases the chance of spontaneous resolution extremely [16-19]. Patients showing a benign, favourable course, and mild symptoms or trivial laboratory findings three years after the initial diagnosis were determined to have a stable or benign disease course.
Ninety-two patients without ocular sarcoidosis were compared to eighty patients with ocular disease. Data variables were represented by mean ± standard deviation. Statistical differences between patients with and without ocular sarcoidosis were evaluated in regard to prognosis, limited or extensive extrapulmonary organ sarcoidosis, and limited or diffuse endobronchial involvement. The X2 test was used for categorical variables as appropriate. Logistic regression was applied to determine the effect of age, gender, and splenic disease on prognosis. Kruskal-Wallis test and Bonferronni corrected two way Mann-Whitney test were used for comparision of the groups. Student’s t-test was done to compare the differences between serum ACE, serum Ca, 24h urinary Ca values, PFT, and DLCO/Va percentages (actual/predicted) between the two groups. Statistical analysis was evaluated using software (SPSS 22.0 version). A threshold p value less than 0.05 was accepted for statistical significance.
Results
Study population consisted of 172 sarcoidosis patients (96 female, 55.8%) with a mean age of 46.2±18.6 years. Eighty patients (80/172, 46.5%) had ocular sarcoidosis. Dermographic distribution, clinical features of the patients, distribution of ocular disease according to stage, and the incidence of time onset for ocular involvement is depicted in Table 1. Tuberculine test was negative in 72% of the patients. Mucosal abnormalities compatible with sarcoidosis was observed in 58% of the cases by bronchoscopy. The most frequent lesion was miliary nodules observed in 36% of the patients, followed by nodular (28%), and erythematous lesions (24%) while 18 percent had mixed type of lesions. Noncasefied granulomatous inflammation was identified from the endobronchial biopsy samples in 98 patients (98/172, 68.6%) while the biopsy was positive in 46 (46/172, 32.5%) patients with a normal appearing airway mucosa. Culture of bronchial lavage for bacteria, mycobacteria, and fungus was negative in all patients. The patients underwent fundoscopic, biomicroscopic, and tonometric examination for the identification of ocular involvement. Anatomic localization of ocular sarcoidosis is shown in table 2. Anterior uveitis was the most common (35%) ocular manifestation followed by posterior and panuveitis. Ocular involvement was the presenting symptom in 12 patients (15%) while 14 subjects (17.5%) had asymptomatic eye involvement. Most frequent symptom was red eye occuring in 24.8% of the patients. Anterior uveitis was the most common site (38.4%) of involvement. Conjunctival biopsy was performed in 38 (26 cases with visible lesions conjunctival lesions) and lacrimal biopsy was done in 24 patients. The positivity of conjunctival biopsy was 100% in the presence of visible lesions while the yield of random conjunctival biopsy was 58% in patients without visible lesions.
Diagnostic histopathologic tissue was obtained by FOB in 68%, by skin biopsy in 34%, via mediastinoscopy in 19%, and by various organ biopsies in 64% of the patients. None of the subjects developed any significant complications associated with the biopsy procedures. Logistic regression with Krukal-Wallis test and Bonferronni corrected two way Mann-Whitney test revealed no statistical difference of age and gender on prognosis. Serum, and 24 h urinary Ca values were not distinct (p<0.9, p<0.11) (Table 1) between the two groups. Serum ACE was (p<0.05) higher in patients with ocular sarcoidosis. There was no significant difference between the FEV1, FVC, and TLC percentage values of the two groups while DLCO/VA was lower (p<0.05) in patients with ocular involvement (Table 1).
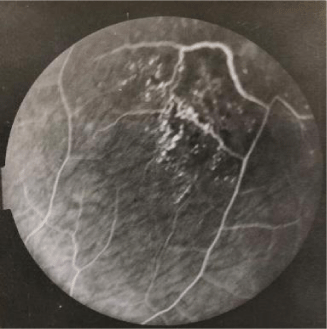
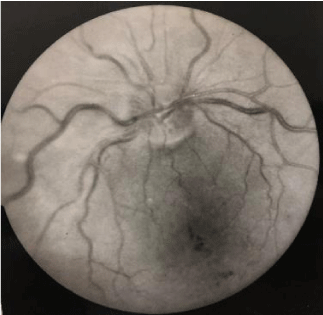
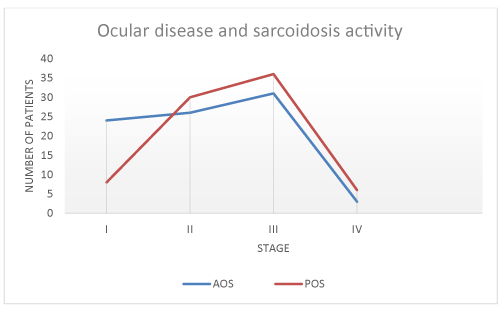
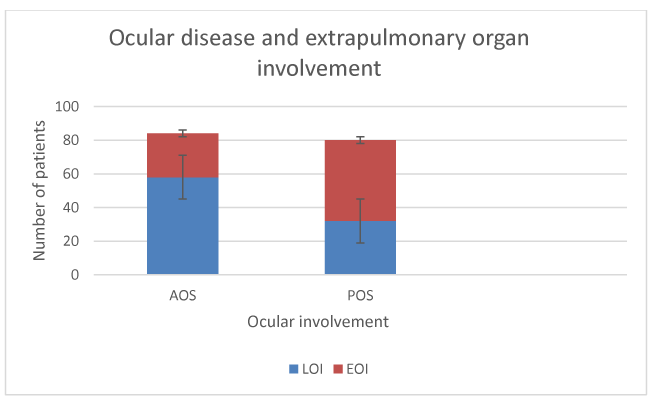
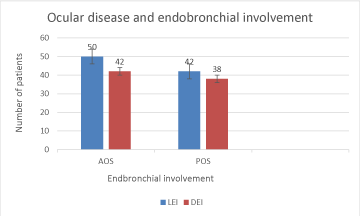
Fluorescein angiography findings of two patients are depicted in figures 1,2. Association of eye sarcodosis with prognosis, extrapulmonary organ, and endobronchial involvement is shown in Figures 3, 4, and 5. The incidence of eye involvement was lowest in stage I patients and showed an increasing trend in regard to stage (Table 1) and was most common in stage III patients. Prevalance of persistent chronic sarcoidosis was significantly higher (p<0.01) in patients with ocular involvement than the patients without eye disease (Figure 1). Extensive extrapulmonary organ involvement (61.6%) was also more frequent in patients with ocular sarcoidosis (Figure 2). Likelihood ratio of extensive extrapulmonary organ involvement was significantly higher (p<0.01) in patients with ocular sarcoidosis. Diffuse endobronchial disease was more common in this group but the difference was insignificant (p<0.24) statistically. Logistic regression analysis revealed a 1.6 times worse prognosis and a 1.4 times more frequent extensive extrapulmonary organ disease (p<0.05) in patients with ocular sarcoidosis. Of the nineteen patients (19/80, 24%) with ocular sarcoidosis, twelve (12/80, 15%) presented with isolated lung and seven (7/80, 8.7%) with only cutaneous involvement. Fourteen patients (17.5%) with ocular disease were asymptomatic at the initial admission. Ocular sarcoidosis developed exclusively within the six years of the initial diagnosis while no ocular involvement was encountered thereafter. None of the patients with stage 0 disease had ocular sarcoidosis.
Discussion
Clinically evident eye involvement in sarcoidosis is not uncommon occuring in approximately 20% to 30% while uveitis is reported in 30% to 70% and conjunctival nodules are found in 40% of the sarcoidosis patients. The reported incidence of ocular sarcoidosis varies considerably from 11.8 to 79 percent [2-4,7]. Ocular sarcoidosis may develop at any stage of sarcoidosis as it may be asymptomatic or appear as the presenting manifestation of sarcoidosis that may involve any compartment of the eye [2-4,20-23]. Currently, data relevant to the clinical and prognostic features of ocular sarcoidosis is scarce because of the asymptomatic course, the variable incidence of eye involvement, and to the different diagnostic criteria used. Absence of distinct and definitive clinical features pointing out to ocular involvement may have also contributed to the variable incidence of ocular sarcoidosis. The results of our study reveal that ocular involvement is a crucial aspect of sarcoidosis. Identification of ocular disease may be useful to predict the prognostic outcome and extrapulmonary organ involvement in sarcoidosis patients. Although statistically insignificant, the higher incidence of endobronchial involvement in patients with ocular disease may eventually point out to the presence of endobronchial sarcoidosis that appears as a crucial clinical perspective of our study.
Despite the high incidence of ocular sarcoidosis, most of the patients do not undergo eye examination. This may be partly due to the asymptomatic nature of eye sarcoidosis, partly due to the lack of specific laboratory findings revealing ocular involvement, and partly due to the difficulties for the identification of ocular disease. Frequency of ocular involvement as the initial manifestation of sarcoidosis is highly variable from 1.5 to 87 percent depending upon the diagnostic criteria used, medical specialty of the clinicians, and genetic features of the patient population [8,22,23]. Incidence of ocular involvement as the presenting manifestation of sarcoidosis was 11.5 percent among our patients. Despite this low incidence, the results of our study reveal that opthalmologic examination should be one of the primary initial diagnostic steps in the evaluation of sarcoidosis patients not only for the identification of ocular disease but also for many aspects of sarcoidosis including the prognostic outcome, extrapulmonary organ, and endobronchial involvement. Even though the method of sarcoidosis diagnosis has been established, the diagnosis is never secure. There must be evidence of granulomatous inflammation in at least two organs for an accurate diagnosis of sarcoidosis [10,11]. In our study, eighteen patients presented with noncasefied granulomatous inflammation of a single organ thereby leading to an equivocal sarcoidosis diagnosis. Identification of ocular disease in these patients confirmed the final diagnosis. Recognition of ocular sarcoidosis appears to be extremely useful to confirm the final diagnosis for patients with an indecisive sarcoidosis presentation due to single organ involvement.
It is well-kown that sarcoidosis is a benign and self-limiting disease that leads to chronic, persistent, or fibrotic disease only in a minority of the patients. Identification of such patients carrying risk factors for a severe prognostic disease course that eventuates in fibrosis is the hallmark for the follow-up and the treatment of sarcoidosis patients. The presence of ocular involvement may be a relevant clinical marker for a severe prognostic outcome in sarcoidosis patients. This finding may be explained by the fact that the granuloma burden in sarcoidosis is the hallmark of persistent and progressive disease [24-29]. The adverse outcome in patients with ocular sarcoidosis is primarily due to the presence of high granuloma burden because as the number of involved organs increase the granuloma load also grows. Serum ACE produced in the epitheloid cells of the sarcoid granuloma reflects the total granuloma load in sarcoidosis [27,28]. The excessive granuloma burden partly caused by ocular disease in our study is verified by the presence of higher serum ACE levels in these patients. The aim of sarcoidosis treatment is granuloma supression thereby preserving organ function and attenuation of fibrosis [29-31]. The kinetic chain of events of granuloma formation, evolution, and distribution is the hallmark for the prognostic outcome of sarcoidosis patients. The inconsistent propensity of granuloma formation and dissemination is the foremost pathologic mechanism liable for the differences of prognosis and organ involvement between individual patients [32-34]. Our study reveals that the transformation of the granuloma burden is the most distinctive marker of sarcoidosis outcome while the impact grade or the intensity of influence of granuloma burden for each organ is uncertain. The granuloma load is the decisive factor for prognosis and extrapulmonary organ involvement in the sarcoidosis patient. The frequency of endobronchial involvement was higher in patients with ocular sarcoidosis. Although this finding was not statistically significant, the presence of eye disease may point out to the existence of endobronchial granulomas thereby indicating a crucial and a distinctive feature for the clinicians.
There are some potential limitations of our study. The first limitation is the small sample size. Second, the clinical presentation and the outcome of sarcoidosis patients show a considerable variation due to the genetic factors. African Americans with sarcoidosis have a higher likelihood of developing ocular involvement compared to Caucasians while race may also influence the age of onset of uveitis [6-8]. Our study group consisted of exclusively Caucasian people. It is well-known that sarcoidosis shows a great variability for clinical manifestations and prognostic outcome in regard to genetic background [10,35]. Third, the follow-up interval may be considered short. It is well known that most of the prognostic risk factors for progressive disease become appearent within the two years of diagnosis and patients with a persistent disease after five years from diagnosis are designated to have chronic disease [11,14-17]. The mean follow up period was approximately nine years in this study. Fourth, the bronchoscopic biopsy samples may be considered inadequate for the identification of the non-casefied granulomatous inflammation especially in the normal mucosa while deeper mucosal biopsy sampling would be necessary for diagnosing endobronchial involvement. The inadequate depth of mucosal biopsies may be the cause for the low frequency of endobronchial involvement incidence in patients with ocular sarcoidosis. The variable incidence of eye involvement in sarcoidosis is attributable to patient features, to the extent of opthalmologic examination, and to the diagnostic criteria used for ocular sarcoidosis. These factors may have inversely influenced the incidence of ocular involvement in our study. Further studies with larger sample sizes, different genetic backgrounds, and more heterogenous patient populations would be useful to determine the exact association of ocular sarcoidosis with the clinical features, the prognostic outcome, the extrapulmonary organ, and the endobronchial involvement in sarcoidosis patients.
The results of our study suggest that ocular involvement in sarcoidosis is a crucial risk factor for progressive disease. Extensive organ involvement is more common in patients with ocular sarcoidosis. The incidence of eye involvement from sarcoidosis is highly variable because patients are asymptomatic, different clinical criteria for the diagnosis of ocular sarcoidosis are used, and in most of the patients opthalmologic examination is not performed. These factors may have lead to a low incidence of clinically detectable of ocular sarcoidosis. The accurate distinction between persistent chronic and self-limited benign sarcoidosis is difficult in clinical practice. Progressive sarcoidosis does not always lead to advanced or fibrotic disease but the diagnosis of such phenotypes appears to be the pivotal step for treatment. Identification of ocular involvement by opthalmologic examination is a useful, a simple, and a noninvasive clinical tool to establish the diagnosis and to predict the chronic outcome in sarcoidosis. Optimal management of sarcoidosis depends upon determining the patients with a risk factor for persistent disease course which is the most fundamental treatment goal. Eye examination is a practical clinical enterprise for a meticulous follow-up and to determine the treatment options in sarcoidosis patients as a preliminary application while confirming the diagnosis of sarciodosis in equivocal cases with only one organ involvement. Despite the statistically insignificant data, patients with ocular disease may have with a higher frequency of bronchial granulomas that may be an important clinical finding as a conspicous evidence for endobronchial involvement.
Author Contributions
Halil Yanardag has contributed to patient and data evaluation.
Cuneyt Tetikkurt has contributed to manuscript preparation and to study design.
Muammer Bilir has contributed to patient follow-up and statistical analysis.
Halit Pazarlı has performed the ophthalmologic examination, diagnosis of eye involvement, and the follow-up of patients with ocular sarcoidosis.
- Crick RP, Hoyle C, Smelle H (1961) The eyes in sarcoidosis. Br J Opthalmolol. 45:461-81.
- Pasadhika S, Rosenbaum JT (2015) Ocular Sarcoidosis. Clin Chest Med. 36: 669-83.
- Ohara K, Okubo A, Sasaki H, Kamata K (1992) Intraocular manifestations of systemic sarcoidosis. Jpn J Ophthalmol. 36:452-7. Link: https://goo.gl/LVxg1S
- Heiligenhaus A, Wefelmeyer D, Wefelmeyer E, Rösel M, Schrenk M (2011) The eye as a common site for the early clinical manifestation of sarcoidosis. Ophthalmic Res. 46: 9-12. Link: https://goo.gl/y46eWr
- Atmaca LS, Atmaca-Sönmez P, Idil A, Kumbasar OO, Celik G (2009) Ocular involvement in sarcoidosis. Ocul Immunol Inflamm. 17: 91-4.
- Birnbaum AD, Oh FS, Chakrabarti A, Tessler HH, Goldstein DA (2011) Clinical features and diagnostic evaluation of biopsy-proven ocular sarcoidosis. Arch Ophthalmol. 129: 409-13. Link: https://goo.gl/orraj9
- Rothova A (2000) Ocular involvement in sarcoidosis. Br J Opthalmol. 84: 110-6.
- Dana MR, Merayo-Lloves J, Schaumberg DA, Foster CS (1996) Prognosticators for visual outcome in sarcoid uveitis. Ophthalmology. 103: 1846-53. Link: https://goo.gl/6qTuda
- Herbort CP, Rao NA, Mochizuki M (2009) members of Scientific Committee of First International Workshop on Ocular Sarcoidosis. International criteria for the diagnosis of ocular sarcoidosis: results of the first International Workshop On Ocular Sarcoidosis (IWOS). Ocular Immunol Inflamm. 17: 160-9. Link: https://goo.gl/cKK2Qh
- Statement on sarcoidosis (1999) Am J Respir Crit Care Med. 160: 736-55.
- Judson MA (2008) The diagnosis of sarcoidosis. Clin Chest Med. 29: 415-27.
- DeRemee RA (1983) The roentgenographic staging of sarcoidosis: historic and contemporary perspectives. Chest. 83: 128-32. Link: https://goo.gl/nGqYyb
- Lung function testing (1991) Selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 144:1202-18. Link: https://goo.gl/7FzqK6
- Mañá J, Salazar A, Manresa F (1994) Clinical factors predicting persistence of activity in sarcoidosis: a multivariate analysis of 193 cases. Respiration. 61: 219-25. Link: https://goo.gl/3hfy5N
- Neville E, Walker AN, James DG (1983) Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 52:525-33. Link: https://goo.gl/XPLgXE
- Chappell AG, Cheung WY, Hutchings HA (2000) Sarcoidosis: a long-term follow up study. Sarcoidosis Vasc Diffuse Lung Dis. 17: 167-73. Link: https://goo.gl/uJ9biE
- Judson MA, Baughman RP, Thompson BW (2003) Two year prognosis of sarcoidosis: the ACCESS experience. Sarcoidosis Vasc Diffuse Lung Dis. 20: 204-11. Link: https://goo.gl/UYa64Z
- Baughmann RP,Costabel U, du Bois RM (2008) Treatment of sarcoidosis. Clin Chest Med. 29: 533-48.
- Lazar CA, Culver DA (2010) Treatment of sarcoidosis. Semin Respir Crit Care Med. 31: 501-18.
- Wijsenbeek MA, Culver DA (2015) Treatment of sarcoidosis. Clin Chest Med. 38: 751-67.
- Baughman RP, Lower EE, Kaufman AH (2010) Ocular sarcoidosis. Semin Respir Crit Care Med. 31: 452-62.
- Rizzato G, Angi M (1996) Uveitis as a presenting feature of chronic sarcoidosis. Eur Respir J. 9: 1201-5.
- Groen F, Rothova A (2017) Ocular Involvement in Sarcoidosis. Semin Respir Crit Care Med. 38: 514-522.
- Baughman RP, Culver DA, Judson MA (2011) A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med. 183: 573-81. Link: https://goo.gl/49Uusw
- Govender P, Berman JS, Culver DA (2015) Diagnosis of sarcoidosis. Clin Chest Med. 38: 585-602.
- Judson MA, Thompson BW, Rabin DL, Steimel J, Knattereud GL, et al. (2003) The diagnostic pathway to sarcoidosis. Chest. 123: 406-12. Link: https://goo.gl/HVCtyV
- Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, et al. (2014) Müller-Quernheim J. Sarcoidosis. Lancet. 383: 1155-67.
- Baughmann RP,Costabel U, du Bois RM (2008) Treatment of sarcoidosis. Clin Chest Med. 29: 533-48.
- Petrache I, Moller DR (2006) Mechanisms of Therapy for Sarcoidosis. Sarcoidosis: Baughman RP (ed). New York. Taylor & Fran cis Group. 671-87. Link:
- Lynch JP, Kazerooni EA, Gay SE (1997) Pulmonary sarcoidosis. Clin Chest Med. 18: 755-86.
- Lazar CA, Culver DA (2010) Treatment of sarcoidosis. Semin Respir Crit Care Med. 31: 501-18.
- Marlies SW, Culver DA (2015) Treatment of sarcoidosis. Clin Chest Med. 36: 751-67.
- Yanardag H, Tetikkurt C, Bilir M, Yılmaz E (2017) Association of HLA antigens with the clinical course of sarcoidosis and familial disease. Monaldi Archives for Chest Disease. 87: 79-84. Link: https://goo.gl/fWEFme
- Tetikkurt C, Yanardag H, Pehlivan M, Bilir M (2017) Clinical features and prognostic significance of splenic involvement in sarcoidosis. Monaldi Archives for Chest Disease. 87: 135-9. Link: https://goo.gl/JfbaLh
- Fingerlin T, Hamzeh N, Maier LA (2015) Genetics of Sarcoidosis. Baughmann RP, Culver DA(eds). Clin Chest Med. 36: 569-84.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
