Archives of Renal Diseases and Management
A Rare Case of Secondary Membranous Nephropathy
Department of Nephrology, Burjeel Royal Hospital, Al Ain, United Arab Emirates
Author and article information
Cite this as
Prabhu H. A Rare Case of Secondary Membranous Nephropathy. Arch Renal Dis Manag. 2025; 10(0): 001-003. Available from: 10.17352/2455-5495.000048
Copyright License
© 2025 Prabhu H. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Membranous Nephropathy (MN) is a leading cause of nephrotic syndrome in adults. It is broadly classified into primary (idiopathic) and secondary forms, based on underlying aetiology. Among the secondary causes, infections— particularly hepatitis B and hepatitis C viruses—play a significant role in disease pathogenesis.
Membranous Nephropathy (MN) is a leading cause of nephrotic syndrome in adults. It is broadly classified into primary (idiopathic) and secondary forms, based on underlying aetiology. Among the secondary causes, infections— particularly hepatitis B and hepatitis C viruses—play a significant role in disease pathogenesis.
Hepatitis C virus (HCV), a major cause of chronic liver disease, is a blood-borne pathogen known to have multiple extrahepatic manifestations, including renal involvement. Its association with various glomerular diseases, such as membranous nephropathy, underscores the importance of considering HCV in the differential diagnosis of secondary MN.
Background
A 35-year-old Egyptian male (MRN: 606016783) presented to the nephrology outpatient clinic with generalised oedema and frothy urine. He had previously been evaluated at an outside hospital, where he was diagnosed with adult-onset nephrotic syndrome and empirically started on corticosteroid therapy.
Despite 16 weeks of steroid treatment, the patient showed only a partial response. Upon evaluation at our center, his serum creatinine was within normal limits at 79 µmol/L. Urinalysis revealed proteinuria of 1147 mg/day along with microscopic hematuria.
Complement levels, Antinuclear Antibody (ANA) profile, anti-dsDNA, Anti MPO, anti-PR3, HIV, and hepatitis B serologies were all within normal ranges. The serum anti-phospholipase A2 receptor (PLA2R) antibody was negative. Given the patient’s history of hepatitis C infection, cryoglobulin testing was conducted and returned negative. Due to persistent proteinuria, a renal biopsy was performed.
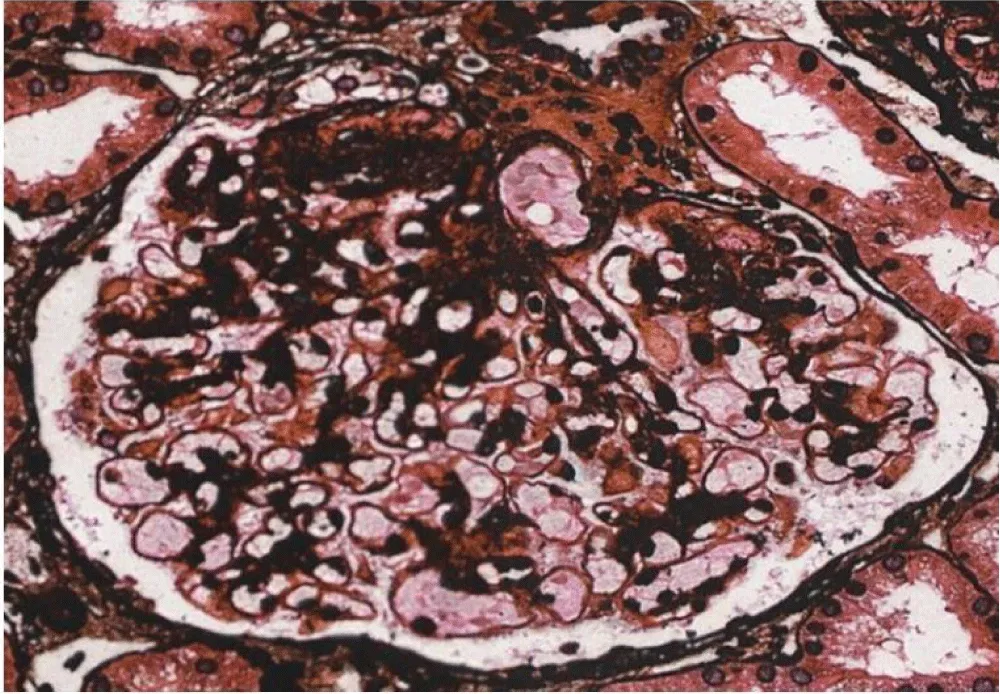
Light Microscopy demonstrated nine glomeruli on light microscopy, one of which was globally sclerosed. There was a global thickening of the capillary walls with segmental duplications as shown in Figure 1. The mesangium appeared normal, and approximately 10% tubular interstitial atrophy was noted.
Immunofluorescence showed diffuse granular positivity along the capillary walls and mesangium for IgG (2+) and C3 (1+).
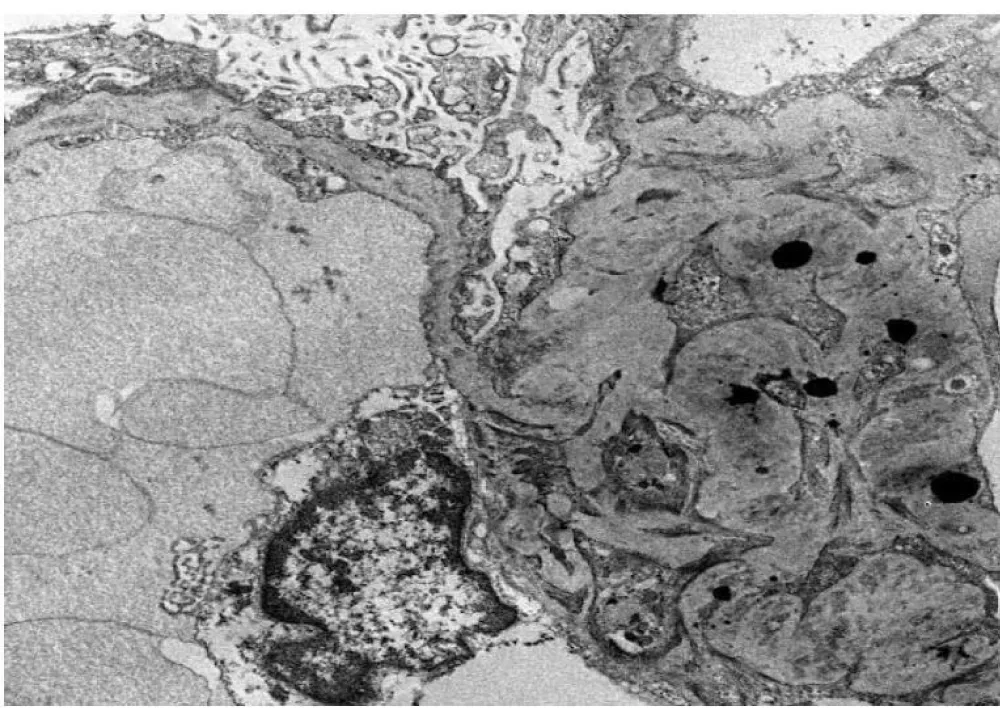
Electron microscopy revealed segmental podocyte microvilli formation and 100% foot process effacement. Discrete and confluent subepithelial and intramembranous electron-dense deposits were observed in an advanced state of lysis, as shown in Figure 2. There was a mild increase in the mesangial matrix with frequent mesangial deposits. No tubuloreticular inclusions were identified.
The findings were consistent with secondary membranous glomerulopathy associated with focal and segmental glomerulosclerosis. Tissue staining for PLA2R, IgG4, and THSD7A was negative. In addition, serum PLA2R antibody was tested three times and was persistently negative.
A comprehensive evaluation for secondary causes was conducted. A chest Xray showed no abnormalities. Hepatitis C virus RNA PCR was undetectable, though anti-HCV antibody remained positive, indicative of a prior infection. Serum PSA and Alpha-Fetoprotein (AFP) levels were within normal limits. Upper gastrointestinal endoscopy, performed to rule out gastric malignancy, was unremarkable. A Computed Tomography (CT) scan of the chest, abdomen, and pelvis revealed no abnormalities.
No definitive secondary cause for membranous nephropathy was identified. However, the possibility of hepatitis C antibody-associated secondary membranous nephropathy was considered, based on the patient’s past infection.
Discussion
Hepatitis C can affect the kidneys in multiple ways. Among all types of glomerulonephritis, membranoproliferative glomerulonephritis (MPGN) is the most common [1]. Other manifestations of hepatitis C on the kidneys include mixed cryoglobulinemia, membranous nephropathy, focal segmental glomerulosclerosis, proliferative glomerulonephritis, and immunotactoid glomerulopathies [2].
It can even affect kidney transplant recipients. A study by Yamabe et al. showed that 8% of membranous nephropathy patients were anti-HCV-positive or HCV RNA-positive, while less than 1% of patients with other types of glomerulonephritis (excluding MPGN) had similar findings [3]. In another study of post-renal transplant patients who developed membranous nephropathy, the prevalence of hepatitis C was higher compared to non-hepatitis C patients [4].
Membranous nephropathy is one of the recognized renal manifestations of hepatitis C infection. Studies from Egypt have shown that in patients with glomerulopathy, the prevalence of HCV antibodies was significantly higher (38%) than among healthy blood donors (16%).
The pathogenesis of hepatitis C-related membranous nephropathy is mainly immune-complex-mediated. Following hepatitis C infection, immune activation involving T cells, B cells, and monocytes leads to the formation of immune complexes, which circulate in the bloodstream and become deposited in the glomeruli, primarily along the glomerular basement membrane [5].
These immune complexes deposit just beneath the podocytes lining the glomerular capillaries, causing thickening of the glomerular basement membrane. Complement activation ensues, generating C5a and C3b, which enhance glomerular inflammation. This leads to the recruitment of neutrophils and macrophages, resulting in podocyte injury and disruption of the glomerular filtration barrier, ultimately causing proteinuria [6].
The clinical manifestations are similar to idiopathic membranous nephropathy. Previously, interferon was used for treatment, but with the advent of directacting antivirals (DAAs), treatment strategies have evolved.
Our patient had hepatitis C infection treated successfully over 10 years ago, with a subsequent sustained virological response. He later developed nephrotic syndrome, and a kidney biopsy confirmed secondary membranous nephropathy. The workup for other secondary causes—including HIV, hepatitis B, autoimmune diseases, and malignancies—was negative. HCV RNA PCR was repeatedly negative over a year. Age-appropriate cancer screening, upper and lower GI endoscopies, and cross-sectional imaging of the chest, abdomen, and pelvis were also normal.
Other potential viral co-triggers for immune-mediated kidney injury, such as Epstein-Barr virus (EBV) and SARS-CoV-2, were also considered and ruled out in this case to ensure a thorough evaluation of possible aetiologies. The patient had received full COVID-19 vaccination as per national guidelines, with no adverse events or temporal relationship to kidney symptoms noted.
In our case, the patient’s secondary membranous nephropathy is likely related to a history of Hepatitis C infection and subsequent Immune- related glomerulopathy. The proposed mechanism involves the formation of circulating immune complexes composed of viral antigens and host antibodies. These complexes can deposit in the kidneys, triggering complement activation and podocyte injury. Notably, even after successful antiviral treatment and viral clearance, immune complexes formed during the infection may persist in circulation and cause delayed renal damage [7,8]. This slow accumulation over time explains the latent onset of nephropathy years after viral remission. Additionally, persistent immune dysregulation may sustain antibody production and kidney injury.
There is no established consensus on treatment for such cases. However, immunosuppression with steroids and calcineurin inhibitors, as described by Weng, et al. [9] in a similar case of secondary membranous nephropathy, has shown clinical benefit.
He was initially started on an angiotensin receptor blocker. But in view of persistent nephrotic Syndrome and after ruling out all secondary causes, he was started on Low-dose steroids and Calcineurin inhibitors.
The patient responded well to treatment initially and went into complete remission after 6 months of starting treatment. His calcineurin inhibitors were gradually tapered; however, he developed worsening proteinuria.
After 1 year of treatment, the patient is in partial remission. The option for Cyclophosphamide and Rituximab was given but was refused.
His renal function is still well preserved, with proteinuria around 250 mg and haematuria.
Chronological order of events
Conclusion
Our case represents a rare case of Secondary membranous nephropathy in which the patient developed renal manifestations 10 years after treatment of Hepatitis C Infection. Persistent immune activation leads to the production of anti-HCV antibodies, which are deposited in the kidneys and lead to podocyte injury. The patient responded to a combination of steroids and CNIs. This case demonstrates a rare and latent presentation of secondary membranous nephropathy.
Declaration
Ethical approval: This case report was conducted in accordance with the ethical standards outlined in the Declaration of Helsinki. Due to the absence of a formal ethics committee, the study received approval from the Head of the Department of Nephrology at Burjeel Royal Hospital, Al Ain.
Informed consent was obtained from the patient for the publication of this case report, including the use of anonymized data and images. Every effort was made to ensure the patient’s privacy and confidentiality were strictly maintained throughout the research process.
Informed consent: Informed consent was obtained from the Individual whose case is reported. It has been attached.
- Johnson RJ, Gretch DR, Yamabe H, Hart J, Bacchi CE, Hartwell P, et al. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med. 1993;328:465–70. Available from: https://doi.org/10.1056/nejm199302183280703
- Markowitz GS, Cheng JT, Colvin RB, Trebbin WM. Hepatitis C viral infection is associated with fibrillary glomerulonephritis and immunotactoid glomerulopathy. J Am Soc Nephrol. 1998;9:2244. Available from: https://doi.org/10.1681/asn.v9122244
- Yamabe H, Johnson RJ, Gretch DR, Fukushi K, Osawa H, Miyata M, et al. Hepatitis C virus infection and membranoproliferative glomerulonephritis in Japan. J Am Soc Nephrol. 1995;6:220–3. Available from: https://doi.org/10.1681/asn.v62220
- Morales JM, Pascual-Capdevila J, Campistol JM, Fernandez-Zatarain G, Muñoz MA, Andres A, et al. Membranous glomerulonephritis associated with hepatitis C virus infection in renal transplant patients. Transplantation. 1997;63:1634. Available from: https://doi.org/10.1097/00007890-199706150-00017
- Serrano M, Pardo V. Hepatitis C virus-associated membranous nephropathy: pathogenesis and management. J Hepatol. 2013;59(2):356–67.
- Fervenza FC, Markowitz GS. Membranous nephropathy: pathogenesis, diagnosis, and treatment. Clin J Am Soc Nephrol. 2015;10(1):124–30.
- Kattah A. Hepatitis C virus and kidney disease: the clinical consequences of a chronic infection. Hepatology. 2014;60(4):1260–74.
- Díaz M. Hepatitis C virus-associated kidney disease. Am J Kidney Dis. 2016;67(6):890–901.
- Weng Q, Li X, Ren H, Xie J, Pan X, Xu J, et al. Membranous nephropathy associated with hepatitis C virus infection treated with corticosteroids and Ledipasvir-Sofosbuvir: a case report and review of literature. Oncotarget. 2017;8(13):22299–303. Available from: https://doi.org/10.18632/oncotarget.15397
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
