Archives of Renal Diseases and Management
Repercussions of the use of probiotics in the treatment of chronic kidney disease
Luana Azevedo Dourado1, Bianca Braga Gomes1, Cainã Araújo Saraiva1, Sandriny Maria de Almeida Oliveira1 and Sávio Benvindo Ferreira2*
2Professor of Higher Education, Academic Unit of Life Sciences (UACV), Teacher Training Center (CFP), Federal University of Campina Grande (UFCG), 58900-000, Cajazeiras, Paraíba, Brazil
Cite this as
Dourado LA, Gomes BB, Saraiva CA, de Almeida Oliveira SM, Ferreira SB (2023) Repercussions of the use of probiotics in the treatment of chronic kidney disease. Arch Renal Dis Manag. 8(1): 004-011. DOI: 10.17352/2455-5495.000044Copyright
© 2023 Dourado LA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The dysbiotic microbiota is one of the clinical findings in patients with Chronic Kidney Disease (CKD), and this intestinal imbalance is associated with inflammation and oxidative stress. With this, probiotic supplementation presents itself as a possible adjuvant therapy to improve this dysbiosis, due to the contribution to the integrity of the intestinal barrier. In this context, this study aims to explain the relationship between the intestinal microbiota and CKD and the repercussions of the use of probiotics on the prognosis of these patients. For this, a narrative review was developed, using the LILACS, MEDLINE, Embase, and PubMed databases, in which articles available in full from the last 5 years, in Portuguese and English, were included. The indication of probiotics as a complementary therapy in renal patients was verified due to the disturbed intestinal microbiome, which aggravates the patient’s inflammatory state. Thus, the supplementation of probiotics, such as Bifidobacterium longum, Lactobacillus rauteri LRE02, Lactobacillus rhamnosus GG, and Lactobacillus casei shirota, acts in the reduction of nephrotoxic substances derived from metabolism in the dysbiotic intestine, in the reduction of urea levels and creatinine levels, in addition to lower levels of C-reactive protein. Thus, the administration of probiotics has been shown to be a precursor in the modulation of toxins derived from the altered microbiota in these patients. On the other hand, there is still controversy about the use of this therapy, because despite improvements in biochemical manifestations, the effective impact on the preservation of glomerular filtration rate is still poorly understood.
Introduction
Chronic Kidney Disease (CKD) is a public health problem with a global incidence rate of up to 16% and is a prevalent and significant disease worldwide [1]. As a result, patients with CKD can develop several comorbidities, such as hypertension, hyperlipidemia, liver and cardiovascular diseases, gastrointestinal problems and cognitive deterioration, configuring the most common complication among metabolic diseases, such as obesity and type 2 diabetes [2].
Given this context and the vast array of comorbidities that CKD can cause, health systems seek adjuvant treatments for these patients, aiming to mitigate or delay the progression of the disease. Based on this idea, the intestinal microbiota interacts with the kidneys through very complicated mechanisms, including diet, uremic toxins derived from the microbiota, immune-mediated factors, and metabolites such as short-chain fatty acids (SCFAs) [3,4]. Thus, dysbiosis can contribute to the chronic inflammatory process and renal dysfunction [5].
As a result, intervention with probiotics in the intestinal flora can be a promising treatment for patients with CKD, since they can increase the number of beneficial bacteria in the intestine and delay the progression of CKD by regulating chronic inflammation [6,7]. Thus, probiotics have important properties, such as anti-inflammatory, antioxidant, and intestinal modulators [8].
Based on this scenario, it is important to emphasize the relevance of the composition of the microbiota, as it can generate both local effects, acting on the local inflammation process, which can accentuate or even further break the barrier of the intestinal mucosa, and systemic effects, through the generation of circulating metabolites that protect or damage the kidneys and cardiovascular system [9].
Probiotic supplements are composed of natural or genetically modified microorganisms, mainly of the genus lactobacilli, bifidobacteria, and some yeasts, which, when administered in adequate amounts, guarantee the balance of the intestinal flora and confer benefits to the health of the organism [10]. These microorganisms act by producing antibacterial components that inhibit the proliferation of pathogenic bacteria and participate in the immune response, restoring the permeability of the intestinal mucosa, increasing the degradation of residual molecules, and reducing the inflammatory response by blocking receptors [11,12].
Thus, as chronic kidney disease favors the accumulation of uremic toxins that cannot be excreted by failing kidneys, the intestinal environment becomes conducive to the overgrowth of proteolytic bacteria and to increased permeability of the intestinal wall, which induces translocation of bacteria or their fractions in the bloodstream, accelerating systemic inflammation [13,14]. Such a situation generates the activation of the immune system and promotes the production of inflammatory factors so this persistent immune activation is an important risk factor for the progression of CKD [15,16].
In this sense, the reduction of traditional uremic toxins, related to the imbalance of the microbiota in patients with CKD, together with a nutritional approach, represent a relevant intervention, since the association of a low-protein diet with prebiotics and probiotics potentiates the reduction of uremic toxins [17,4]. Therefore, the use of probiotics has been encouraged to improve the quality of life of patients with CKD, in order to increase urea metabolism and reduce the production of uremic toxins [18].
In view of this perspective, the present study aims to carry out a review on the use of probiotics in chronic kidney patients, in order to identify the relationship between the intestinal microbiota and chronic kidney disease and the repercussions of the use of probiotics on the quality of life and prognosis of these patients.
Methodology
Characterization of the research
It is a qualitative and descriptive literature review, in which a critical compilation of articles is made that discuss a subject, that is, it is an analytical text of the ideas studied on the theme chosen for the work. [19]. In order to construct the research question, the PICO (Population, Intervention, Control, and Outcomes/Outcome) strategy was used, resulting in the research question: “What are the repercussions of the use of probiotics in the treatment of chronic kidney patients?”
Conducting the research
The review was carried out during the months of May to August 2023, in the electronic databases LILACS, MEDLINE, Embase, and PubMed, aimed at indexing journals and scientific articles. The keywords provided by the Health Sciences Descriptors (DeCS) and the Boolean operators used in the search were: “probiotics” OR “probiotics” AND “renal insufficiency, chronic” OR “renal insufficiency, chronic” AND “treatment outcome” OR “treatment result”, being the search languages Portuguese and English, involving articles published in the last 5 years.
From this, the works were selected by title, followed by reading the abstract and then the full text, if it made reference to the use of probiotics in chronic kidney disease. Exclusion criteria were duplicate studies, review articles, and articles involving animals, and also those after being read in full, their content did not answer the question of this investigation.
Summary of results
From the search criteria, 99 articles were filtered and, at first, reviews and studies involving animals were excluded, followed by other articles excluded by title and abstract and, finally, after a complete reading, 9 articles were selected to compose the state of the art. With this, the analysis of the theoretical foundation of each study was carried out, as well as the observation of the general characteristics of the articles, highlighting the most relevant and debated points to be synthesized in the current narrative review, as shown in Chart 1.
As it is a review article, it was not necessary to submit the study to an ethics and research committee, as the analysis was based on secondary data already published in other articles.
Development
Relationship of intestinal microbiota with chronic kidney disease
The intestinal microbiota is composed of a wide variety of microorganisms that live in a mutually beneficial symbiosis relationship with the host organism, whether bacteria, mostly, viruses, fungi, or protozoa, among others [20]. In addition, it is responsible for several fundamental functions, such as maturation and activation of the immune system in defense against pathogens, in addition to participating in several metabolic pathways and providing nutrients, vitamins, short-chain fatty acids and precursors of uremic toxins [21].
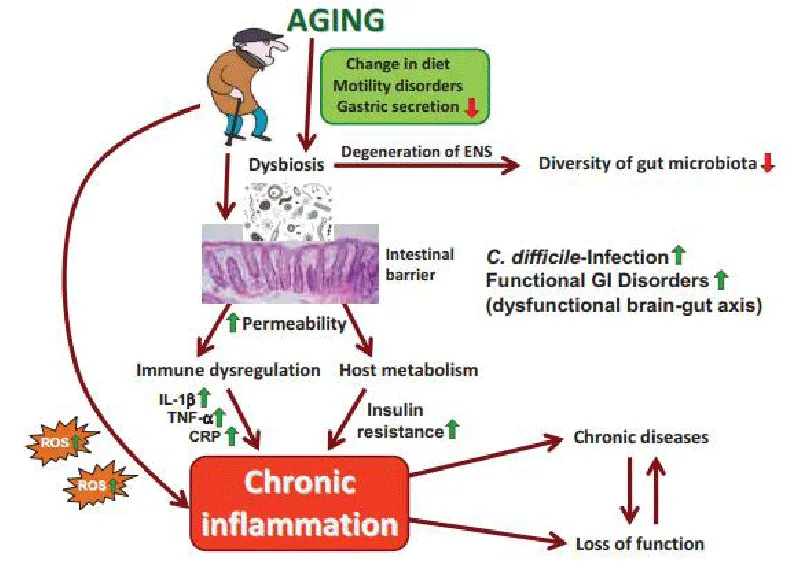
These microorganisms are in a constant dynamic state, being extremely sensitive to exogenous agents, variants of each host, and changes in the environment in which they live. Such factors can lead to a microbial imbalance, called dysbiosis, and to the proliferation of pathogenic bacteria that secrete a large number of bacterial proteins in the blood, stimulating the production of pro-inflammatory cytokines, which generates immune activation and a chronic inflammatory process (Figure 1) [22]. Thus, alteration of the intestinal microbiome can cause inflammation that is related to the alteration of several metabolic pathways in different body tracts, reflecting the development of several diseases, such as chronic kidney disease, or increasing the pro-inflammatory state of this pathology [ 23].
Furthermore, uremia, caused by CKD, increases the levels of urea and ammonia in the Gastrointestinal Tract (GIT), which raises the pH in this region, causing changes in the bacterial proportion of the intestinal microbiota, and characterizing dysbiosis [24]. This alteration in the microbiome occurs due to the elevation of urea in the body, which promotes the overgrowth of proteolytic bacteria [20].
Thus, due to changes in the composition of the microbiota in CKD, bacteria with proteolytic activity are present in greater numbers, producing uremic toxins and other toxic compounds that are responsible for a negative impact on various body functions, such as increased cardiovascular mortality and worsening of the inflammatory process [23]. It is worth adding that bacteria with saccharolytic activity are fewer in number, reflecting the reduced production of short-chain fatty acids and, consequently, the worsening of the chronic inflammatory state of CKD [25].
Benefits of using probiotics in chronic renal patients
Patients with chronic kidney disease are exposed to several aspects that can compromise the intestinal microbiota, such as malnutrition, edema, physical or psychological stress, food restriction, constipation, uremia, and others, which aggravate systemic inflammation and oxidative stress [23].
In addition to these factors, the generation of uremic toxins p-Cresyl Sulfate (p-CS), Indoxyl Sulfate (IS) and Indole 3-Acetic Acid (IAA) may be related to the intestinal flora and markers of progression of chronic kidney disease [20,24]. Regarding the damage caused by these toxins, the accumulation may favor an increase in the permeability of the intestinal wall, which is necessary to prevent microbial toxins or antigens from penetrating systemically, in order to avoid systemic inflammation [20].
Thus, the metabolites are accumulated generating pathogenic bacterial overgrowth, which causes dysbiosis and induces the production of uremic toxins derived from the intestine, causing the progression of CKD. This dysfunction of the intestinal microbiota is harmful to the organism, since the metabolites mentioned cause an increase in reactive oxygen species, stimulating pro-inflammatory secretion of cytokines and a pro-fibrotic response that aggravates renal dysfunction. That is, the decrease in the Glomerular Filtration Rate (GFR) favors the formation of oxidative metabolites, triggering renal tubulointerstitial fibrosis and glomerulosclerosis, which, in turn, further compromises the GFR [26].
Regarding the association of the dysbiotic environment with indicators of the severity of chronic kidney disease, altered levels of the microbiota genera Parasutterella, Lactobacillus, Paraprevotella, Clostridium sensu stricto, and Desulfovibrio have been shown to be correlated with a decrease in the glomerular filtration rate, for example (Figure 2) [27]. In this sense, patients with CKD exhibit a significant decrease in the structure of the fecal microbiota, with a lower abundance of the genus Akkermansia, which is related to systemic inflammation in CKD.
It was also observed that probiotic supplementation has been indicated as an adjuvant therapy to improve the balance of the intestinal microbiota, due to the contribution to the integrity of the intestinal barrier and metabolic control [23]. Accordingly, the benefits of using probiotics in the control and modulation of microbiota-derived and proatherogenic toxins in patients with chronic kidney disease have been demonstrated [20].
In this scenario of seeking intestinal balance, probiotics are natural or genetically modified microorganisms that express specific exogenous enzymes capable of surviving stomach acid and bile, increasing concentrations of symbionts in the colon, and generating health benefits [23]. Accordingly, probiotics are defined as live microorganisms that, when administered in adequate amounts, confer a health benefit on the host [20].
In comparison to harmful microbial actions, the effects of probiotics involve changes in intestinal pH, antagonism of pathogens through the production of antibacterial components, and competition for available nutrients [23]. Thus, the consumption of probiotics, in association with extruded sorghum cereals, was identified as a potential reducer of serum levels of C-reactive protein, in addition to increasing the total antioxidant capacity in patients with Chronic Kidney Disease [25]. Therefore, probiotics may play a therapeutic role in delaying the progression of kidney disease [26].
In addition, as a vicious circle, the high intraluminal concentration of urea is related to the growth of bacteria with urease activity that can cause increased plasma levels of urea [23]. Then, ammonia resulting from bacterial activity can be absorbed before being converted to ammonium hydroxide and entering the urea cycle, thus contributing to free radical production, pro-fibrotic damage to mesangial cells, apoptosis, and disruption of the intestinal barrier.
Strains used in probiotic therapies
A randomized, double-blind, placebo-controlled study investigated the role of a probiotic formulation from the strains Bifidobacterium longum and Lactobacillus rauteri LRE02 in reducing traditional uremic toxins derived from the microbiota in patients with advanced CKD, concluding that probiotics, in association with a low-protein diet, they generate remarkable effects in the attenuation of oxidative stress [20].
A once-daily dose of 350 billion CFU of Lactobacillus rhamnosus GG has also been shown to improve intestinal dysbiosis and attenuate the production of gut-derived uremic toxins [26]. So, probiotics can act as part of the therapeutic process in delaying the progression of chronic kidney disease.
Corroborating the probiotic therapeutic competence in reducing levels of these metabolites, a study revealed that the consumption of a symbiotic meal for 7 weeks, including extruded sorghum and the probiotic Bifidobacterium longum BL-G301, reduced levels of p-CS and IS toxins, colonic pH and urea in hemodialysis patients. That is, there is a probiotic contribution to the reduction of systemic inflammation and oxidative stress [24].
Regarding the indicators of reduction of nephrotoxicity, according to a randomized clinical study, a dose of 16 billion CFU per day of Lactobacillus casei shirota, administered for two months in patients with CKD in stages 3 and 4, was able to reduce urea levels [23]. Another study indicated that the use of synbiotics may be promising to contribute to the improvement of anemia in chronic renal patients [28].
Furthermore, regarding the clinical parameters, one of the analyzed alterations most correlated with chronic kidney disease was the reduction of the genera Akkermansia, Lactobacillus, Parasutterella, and Clostridium IV in the microbiota of chronic kidney patients. This finding suggests that mainly Akkermansia and Lactobacillus are promising diagnostic biomarkers for CKD, contributing to the understanding of the role of the microbiota in clinical criteria and, probably, in therapeutic actions [27].
It was also observed that after treatment with Lactobacillus rhamnosus GG, analysis of the fecal microbiome showed a reduction in the diversity and relative abundance of pathogenic proteobacteria compared to the placebo group (0.76 ± 0.32% versus 18.37 ± 9.19 %). Furthermore, gram-positive anaerobes commonly higher in a healthy gut were increased in the probiotic group compared to placebo, suggesting that probiotics may alter the structure of bacterial phyla in this microbiome [26].
Regarding the control of harmful substances, beneficial effects of the use of probiotics were detected in the context of chronic kidney disease, involving the prevention of the increase in the serum level of Lp-PLA2. In addition, the study was able to conclude the reduction in the dose of antihypertensive drugs and loop diuretics observed in the group that used probiotics, using a probiotic composition of 5 x 109 of Bifidobacterium longum and 1 x 109 Lactobacillus rauteri in the formulation, with improvement blood pressure and extracellular fluid volume balance. It was also observed that 17% of patients in the probiotic group required renal replacement therapy, while 50% of patients in the placebo group required such therapy [20].
Although there are indications of possible beneficial repercussions in the use of probiotics to alleviate the cardiovascular impairment of chronic renal patients, there were controversial results, as in the research that used 9×1013 colony-forming units per day of Streptococcus thermophilus, Lactobacillus acidophilus and Bifidobacteria longum for 3 months, and concluded that there are no significant differences, in the short term, in the level of the trimethylamine-N-oxide metabolite, which may favor the development of cardiovascular diseases [23].
Controversies in the administration of probiotics as adjunctive therapy
Studies evaluating the effectiveness of probiotics in CKD indicate different results and advise that probiotic therapy should be chosen with consideration in the case of patients on hemodialysis, demonstrating that this adjuvant therapy in patients with CKD calls for further studies [23]. Accordingly, it has been shown that the effect of intestinal dysbiosis on the production or pathogenesis of inflammatory factors in CKD has not been extensively studied so far [27].
Although several benefits linked to the use of probiotics in chronic renal patients have been reported, challenges and controversies regarding clinical benefits still persist. Such doubts can be exemplified in a study in which, after 3 months of probiotic supplementation, no benefits were observed in biochemical parameters and inflammatory markers in patients with CKD, although other studies with favorable results were recognized. Such supplementation failure may stem from the unfavorable environment in which the strains were introduced. In this case, patients were divided into probiotic and placebo groups, using 3 capsules of probiotic and placebo per day, respectively, for 3 months. These capsules contained 30 billion live bacteria each, totaling 90 billion Colony-Forming Units (CFU) per day, including Streptococcus thermophilus, Lactobacillus acidophilus and Bifidobacteria longum strains [23].
Due to cases that presented therapeutic failure, such as the one mentioned above, the role of probiotics in CKD is not fully understood. For example, in the case of a randomized clinical trial, despite revealing an improvement in dysbiosis, it obtained similar results in proteinuria levels in the placebo group and the probiotic group. Additionally, the study revealed reduced levels of albuminuria, after 12 weeks of treatment, in the probiotic group, however, at the end of probiotic therapy, the results in this criterion also became similar to those of the placebo group [26].
Thus, despite the promising results of the use of probiotics in the correction of dysbiosis, the exact mechanism of how probiotics contribute to kidney health remains, in part, misunderstood. In this sense, an obstacle to conclusions about studies with probiotics is that there are many variables, such as strain diversity, heterogeneity of the studied population, limited sample size, different experimental designs, different treatment periods, and others, limiting comparisons between studies [23].
Discussion
The intestinal microbiota is responsible for maintaining the Gastrointestinal Tract (GIT) and controlling systemic homeostasis, as a quantitative and/or qualitative imbalance is responsible for the pathogenesis of many diseases, such as chronic kidney disease [11,29]. In this chronic disease, interactions between intestinal dysbiosis and the progression of CKD have been demonstrated, as this causes the accumulation of nitrogenous waste, resulting in disorders of secretion, absorption, and motility of the GIT [18].
Such pathology generates profound changes in the composition of the intestinal microbiome and in the disruption of the structure and function of the intestinal epithelial barrier [30]. These alterations contribute to the formation of a dysbiotic intestine, in addition to promoting the production of toxic bacterial metabolites that can contaminate the peripheral blood and develop endotoxemia [31,32].
After analyzing several studies, it was observed that most of them worked with similar microorganisms, which include bacteria of the Bifidobacterium and Lactobacillus genera, with reported beneficial results, such as a decrease in urea, ammonia, and plasma concentrations of p-cresol and indoxyl sulfate [12,33]. Another benefit reported with the use of probiotic strains is that they probably contribute to the increase in populations of bifidobacteria, known to play a protective role in the barrier of the intestinal mucosa, in addition to helping to reduce pro-inflammatory cytokines and endotoxins [34,35].
These probiotic bacteria have been shown to contribute to immune function, promote vitamin synthesis, and block the growth of other harmful bacteria and possible generators of uremic toxins. Such results support the finding that indicates improvements in the maintenance of the glomerular filtration rate from the therapeutic consumption of the formulations, which reduces the levels of toxic products [3,5,36]. The most widely studied uremic retention solutes derived from microbial metabolism that were associated with an increased risk of kidney disease progression were indoxyl sulfate, p-cresyl sulfate and trimethylamine-N oxide (TMAO) [37,38].
In addition, an important biomarker of inflammation, CRP, is noted, which significantly increases in patients with CKD compared to healthy individuals. That said, many studies have shown that probiotic therapy could decrease the inflammatory state of these patients, while in others the results were irrelevant [29].
In this scenario, despite the questions and controversies in some studies, it is understood that probiotics are associated with the improvement of the host’s immune response and inflammatory state. So, it is important to consider, in the context of CKD, that intestinal microbial alterations are characterized by bacteria that present urease-forming enzymes, uricase, indole and p-cresol [39,40]. It is therefore understood that uremic retention solutes are toxic metabolites, difficult to eliminate, and associated with impaired renal function and systemic inflammation [41-44].
Thus, studies show that there are several correlations between intestinal dysbiosis and renal dysfunction, which indicates remarkable possibilities regarding the beneficial effects of probiotics for the treatment of chronic kidney disease [9,45]. That is, the finding that patients with CKD have high levels of C-reactive protein and uremic toxins triggers the need to better understand the effects of improving the health of the intestinal microbiota in reducing the progression of this disease. Therefore, the mechanisms by which probiotics reduce levels of toxic by-products, mitigating the pro-inflammatory secretion of cytokines and the pro-fibrotic response should be further investigated [46,47].
These mechanisms of action include the production of bacteriocins, competition with pathogenic bacteria for nutrients, blocking adhesion sites for pathogenic bacteria, maintenance of the intestinal barrier, and modulation of the immune response [48].
Another important consideration is the possibility of associating probiotic formulas with prebiotics, with a low-protein diet, or with fiber intake. So, to achieve more accurate results, it is important to investigate and compare the effectiveness of probiotic therapy when used separately or in conjunction with other nutritional interventions [7,18,48]. In line with this, the quantity and quality of proteins and fibers can influence the production of uremic toxins and the composition of the intestinal microbiota [49].
It is worth mentioning that it is important to warn patients about the risks of using probiotics deliberately and without medical indication, because, despite being beneficial bacteria for the intestine, they can cause bacteremia due to an irregular response of the GIT [50,51].
Conclusion
It is concluded that the administration of probiotics has been shown to be beneficial in the control and modulation of toxins derived from the altered microbiota in renal patients, and can be used as an adjuvant therapy. With this, it was observed that probiotics delay the progression of chronic kidney disease, and a possible mechanism of this effect would be the decrease in the formation of nephrotoxic substances derived from the intestine thus attenuating systemic inflammation, although the effects and mechanisms do not fully clarify. of probiotics in the maintenance of the Glomerular Filtration Rate (GFR) and in the clinical repercussions.
On the other hand, there are still controversies about the use of this therapy, because although there are improvements in the biochemical manifestations of renal failure, such as the reduction in serum creatinine and urea concentrations, the effective impact on the preservation of GFR has not yet been fully confirmed or understood, as such a measure requires more reliable exogenous markers.
- Inatomi T, Honma M. Ameliorating effect of probiotics in a rat model of chronic kidney disease. Plos One. 30 mar 2023; 18(3): e0281745. https://doi.org/10.1371/journal.pone.0281745
- Plata C, Cruz C, Cervantes LG, Ramírez V. The gut microbiota and its relationship with chronic kidney disease. Int Urol Nephrol. 2019 Dec;51(12):2209-2226. doi: 10.1007/s11255-019-02291-2. Epub 2019 Oct 1. PMID: 31576489.
- Tian N, Li L, Ng JK, Li PK. The Potential Benefits and Controversies of Probiotics Use in Patients at Different Stages of Chronic Kidney Disease. Nutrients. 2022 Sep 29;14(19):4044. doi: 10.3390/nu14194044. PMID: 36235699; PMCID: PMC9571670.
- Sircana A, De Michieli F, Parente R, Framarin L, Leone N, Berrutti M, Paschetta E, Bongiovanni D, Musso G. Gut microbiota, hypertension and chronic kidney disease: Recent advances. Pharmacol Res. 2019 Jun;144:390-408. doi: 10.1016/j.phrs.2018.01.013. Epub 2018 Jan 31. PMID: 29378252.
- Chen L, Shi J, Ma X, Shi D, Qu H. Effects of Microbiota-Driven Therapy on Circulating Indoxyl Sulfate and P-Cresyl Sulfate in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv Nutr. 2022 Aug 1;13(4):1267-1278. doi: 10.1093/advances/nmab149. PMID: 34905018; PMCID: PMC9340978.
- Wang P, Peng Y, Guo Y, Zhao Y. The efficacy of probiotic preparations on inflammatory cytokines in patients with chronic kidney disease: A protocol for systematic review and meta-analysis. Medicine (Baltimore). 2021 Jul 2;100(26):e26422. doi: 10.1097/MD.0000000000026422. PMID: 34190163; PMCID: PMC8257906.
- Grandson A, Rodrigues F. Impact of the use of probiotics and synbiotics in people with kidney disease: a literature review. Brazilian Journal of Health Review. 2023; 6(3):11606–19. https://ojs.brazilianjournals.com.br/ojs/index.php/BJHR/article/view/60377/43643
- Cavalcanti Neto MP, Aquino JS, Romão da Silva LF, de Oliveira Silva R, Guimarães KSL, de Oliveira Y, de Souza EL, Magnani M, Vidal H, de Brito Alves JL. Gut microbiota and probiotics intervention: A potential therapeutic target for management of cardiometabolic disorders and chronic kidney disease? Pharmacol Res. 2018 Apr;130:152-163. doi: 10.1016/j.phrs.2018.01.020. Epub 2018 Feb 14. PMID: 29410236.
- Beker BM, Colombo I, Gonzalez-Torres H, Musso CG. Decreasing microbiota-derived uremic toxins to improve CKD outcomes. Clin Kidney J. 2022 Jun 15;15(12):2214-2219. doi: 10.1093/ckj/sfac154. PMID: 36381370; PMCID: PMC9664568.
- Jiménez Ortega AI, Martínez García RM, Velasco Rodríguez-Belvis M, Martínez Zazo A, Salas González MªD, Cuadrado-Soto E. Nutrición y microbiota en población pediátrica. Implicaciones sanitarias [Nutrition and microbiota in pediatric population. Health implications]. Nutr Hosp. 2021 Jan 13;37(Spec No2):8-12. Spanish. doi: 10.20960/nh.03349. PMID: 32993303.
- Hsu CN, Tain YL. Chronic Kidney Disease and Gut Microbiota: What Is Their Connection in Early Life? Int J Mol Sci. 2022 Apr 2;23(7):3954. doi: 10.3390/ijms23073954. PMID: 35409313; PMCID: PMC9000069.
- Zheng HJ, Guo J, Wang Q, Wang L, Wang Y, Zhang F, Huang WJ, Zhang W, Liu WJ, Wang Y. Probiotics, prebiotics, and synbiotics for the improvement of metabolic profiles in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2021;61(4):577-598. doi: 10.1080/10408398.2020.1740645. Epub 2020 Apr 24. PMID: 32329633.
- Chao CT, Lin SH. Uremic vascular calcification: the pathogenic roles and gastrointestinal decontamination of uremic toxins. Toxins. 21 dez 2020 [citado 10 ago 2023];12(12):812. https://doi.org/10.3390/toxins12120812
- Sumida K, Lau WL, Kovesdy CP, Kalantar-Zadeh K, Kalantar-Zadeh K. Microbiome modulation as a novel therapeutic approach in chronic kidney disease. Curr Opin Nephrol Hypertens. 2021 Jan;30(1):75-84. doi: 10.1097/MNH.0000000000000661. PMID: 33148949.
- Rysz J, Franczyk B, Ławiński J, Olszewski R, Ciałkowska-Rysz A, Gluba-Brzózka A. The Impact of CKD on Uremic Toxins and Gut Microbiota. Toxins (Basel). 2021 Mar 31;13(4):252. doi: 10.3390/toxins13040252. PMID: 33807343; PMCID: PMC8067083.
- Pisano A, D'Arrigo G, Coppolino G, Bolignano D. Biotic Supplements for Renal Patients: A Systematic Review and Meta-Analysis. Nutrients. 2018 Sep 4;10(9):1224. doi: 10.3390/nu10091224. PMID: 30181461; PMCID: PMC6165363.
- Carlucci A, Cervellera C, De C, Sampayo S, De Vito A, Villafañe C. Contributions of biotechnology to the diagnosis and treatment of chronic kidney disease and common comorbidities. 2020 Jun. https://www1.hospitalitaliano.org.ar/multimedia/archivos/noticias_attachs/47/documentos/114607_105-116-5-8-19-Carlucci-D.pdf
- Felizardo RJF, Watanabe IKM, Dardi P, Rossoni LV, Câmara NOS. The interplay among gut microbiota, hypertension and kidney diseases: The role of short-chain fatty acids. Pharmacol Res. 2019 Mar;141:366-377. doi: 10.1016/j.phrs.2019.01.019. Epub 2019 Jan 10. PMID: 30639376.
- Melnyk BM, Fineout-Overholt E. Evidence-based practice in nursing and healthcare: a guide to best practice. Philadelphia, Pa.; London: Lippincott Williams & Wilkins; 2018.
- De Mauri A, Carrera D, Bagnati M, Rolla R, Vidali M, Chiarinotti D, Pane M, Amoruso A, Del Piano M. Probiotics-Supplemented Low-Protein Diet for Microbiota Modulation in Patients with Advanced Chronic Kidney Disease (ProLowCKD): Results from a Placebo-Controlled Randomized Trial. Nutrients. 2022 Apr 14;14(8):1637. doi: 10.3390/nu14081637. PMID: 35458199; PMCID: PMC9025298.
- Borges NA, Stenvinkel P, Bergman P, Qureshi AR, Lindholm B, Moraes C, Stockler-Pinto MB, Mafra D. Effects of Probiotic Supplementation on Trimethylamine-N-Oxide Plasma Levels in Hemodialysis Patients: a Pilot Study. Probiotics Antimicrob Proteins. 2019 Jun;11(2):648-654. doi: 10.1007/s12602-018-9411-1. PMID: 29651635.
- Eidi F, Poor -reza Gholi F, Ostadrahimi A, Dalili N, Samadian F, Barzegari A. Effect of Lactobacillus Rhamnosus on serum uremic toxins (phenol and P-Cresol) in hemodialysis patients: A double-blind randomized clinical trial. Clin Nutr ESPEN [Internet]. Dez 2018 [citado 11 ago 2023];28:158-64. Disponível em:
- Borges NA, Carmo FL, Stockler-Pinto MB, de Brito JS, Dolenga CJ, Ferreira DC, Nakao LS, Rosado A, Fouque D, Mafra D. Probiotic Supplementation in Chronic Kidney Disease: A Double-blind, Randomized, Placebo-controlled Trial. J Ren Nutr. 2018 Jan;28(1):28-36. doi: 10.1053/j.jrn.2017.06.010. Epub 2017 Sep 6. PMID: 28888762.
- Lopes RCSO, Theodoro JMV, da Silva BP, Queiroz VAV, de Castro Moreira ME, Mantovani HC, Hermsdorff HH, Martino HSD. Synbiotic meal decreases uremic toxins in hemodialysis individuals: A placebo-controlled trial. Food Res Int. 2019 Feb;116:241-248. doi: 10.1016/j.foodres.2018.08.024. Epub 2018 Aug 9. PMID: 30716942.
- Lopes RCSO, de Lima SLS, da Silva BP, Toledo RCL, Moreira MEC, Anunciação PC, Walter EHM, Carvalho CWP, Queiroz VAV, Ribeiro AQ, Martino HSD. Evaluation of the health benefits of consumption of extruded tannin sorghum with unfermented probiotic milk in individuals with chronic kidney disease. Food Res Int. 2018 May;107:629-638. doi: 10.1016/j.foodres.2018.03.004. Epub 2018 Mar 5. PMID: 29580529.
- Treewatchareekorn S, Tungsanga S. Wcn23-0326 effect of lactobacillus rhamnosus gg on gut-derived uremic toxin and gut microbiome in non-dialysis chronic kidney disease patients: a randomized controlled trial. Kidney Int Rep. Mar 2023 [citado 10 ago 2023];8(3):S211—S212. https://doi.org/10.1016/j.ekir.2023.02.475
- Li F, Wang M, Wang J, Li R, Zhang Y. Alterations to the Gut Microbiota and Their Correlation With Inflammatory Factors in Chronic Kidney Disease. Front Cell Infect Microbiol. 2019 Jun 12;9:206. doi: 10.3389/fcimb.2019.00206. PMID: 31245306; PMCID: PMC6581668.
- Kooshki A, Akbarzadeh R, Amin B, Tofighiyan T, Foroumandi E. Synbiotic supplement for treatment of iron deficiency anaemia in haemodialysis patients: A randomized controlled trial. Nephrology (Carlton). 2023 Apr;28(4):234-239. doi: 10.1111/nep.14149. Epub 2023 Feb 14. PMID: 36745046.
- Jia L, Jia Q, Yang J, Jia R, Zhang H. Efficacy of Probiotics Supplementation On Chronic Kidney Disease: a Systematic Review and Meta-Analysis. Kidney Blood Press Res. 2018;43(5):1623-1635. doi: 10.1159/000494677. Epub 2018 Oct 31. PMID: 30380555.
- Madan N, Kaysen GA. Gut Endothelial Leakage of Endotoxin May Be the Source of Vascular Inflammation and Injury in CKD. How Can This Be Targeted? J Ren Nutr. 2018 Jan;28(1):1-3. doi: 10.1053/j.jrn.2017.11.001. PMID: 29249294.
- Dobrek Ł. POTENTIAL THERAPEUTIC OPTIONS TARGETING THE GUT DYSBIOSIS IN CHRONIC KIDNEY DISEASE. Wiad Lek. 2022;75(7):1757-1764. doi: 10.36740/WLek202207127. PMID: 35962694.
- McFarlane C, Ramos CI, Johnson DW, Campbell KL. Prebiotic, Probiotic, and Synbiotic Supplementation in Chronic Kidney Disease: A Systematic Review and Meta-analysis. J Ren Nutr. 2019 May;29(3):209-220. doi: 10.1053/j.jrn.2018.08.008. Epub 2018 Oct 23. PMID: 30366767.
- Belova IV, Khrulev AE, Tochilina AG, Khruleva NS, Lobanova NA, Zhirnov VA, Molodtsova SB, Lobanov VN, Solovieva IV. Colon Microbiocenosis and Its Correction in Patients Receiving Programmed Hemodialysis. Sovrem Tekhnologii Med. 2021;12(5):62-68. doi: 10.17691/stm2020.12.5.07. Epub 2020 Oct 28. PMID: 34796006; PMCID: PMC8596268.
- Fagundes RAB, Soder TF, Grokoski KC, Benetti F, Mendes RH. Probiotics in the treatment of chronic kidney disease: a systematic review. J Bras Nefrol. 2018 Jul-Sep;40(3):278-286. doi: 10.1590/2175-8239-jbn-3931. Epub 2018 Jun 21. PMID: 29958304; PMCID: PMC6533949.
- Bakhtiary M, Morvaridzadeh M, Agah S, Rahimlou M, Christopher E, Zadro JR, Heshmati J. Effect of Probiotic, Prebiotic, and Synbiotic Supplementation on Cardiometabolic and Oxidative Stress Parameters in Patients With Chronic Kidney Disease: A Systematic Review and Meta-analysis. Clin Ther. 2021 Mar;43(3):e71-e96. doi: 10.1016/j.clinthera.2020.12.021. Epub 2021 Jan 30. PMID: 33526314.
- Pan W, Kang Y. Gut microbiota and chronic kidney disease: implications for novel mechanistic insights and therapeutic strategies. Int Urol Nephrol. 2018 Feb;50(2):289-299. doi: 10.1007/s11255-017-1689-5. Epub 2017 Aug 28. PMID: 28849345.
- Snelson M, Biruete A, McFarlane C, Campbell K. A Renal Clinician's Guide to the Gut Microbiota. J Ren Nutr. 2020 Sep;30(5):384-395. doi: 10.1053/j.jrn.2019.11.002. Epub 2020 Jan 9. PMID: 31928802; PMCID: PMC7412595.
- Cosola C, Rocchetti MT, di Bari I, Acquaviva PM, Maranzano V, Corciulo S, Di Ciaula A, Di Palo DM, La Forgia FM, Fontana S, De Angelis M, Portincasa P, Gesualdo L. An Innovative Synbiotic Formulation Decreases Free Serum Indoxyl Sulfate, Small Intestine Permeability and Ameliorates Gastrointestinal Symptoms in a Randomized Pilot Trial in Stage IIIb-IV CKD Patients. Toxins (Basel). 2021 May 5;13(5):334. doi: 10.3390/toxins13050334. PMID: 34063068; PMCID: PMC8147955.
- Yang CY, Tarng DC. Diet, gut microbiome and indoxyl sulphate in chronic kidney disease patients. Nephrology (Carlton). 2018 Oct;23 Suppl 4:16-20. doi: 10.1111/nep.13452. PMID: 30298666.
- Liu T, Wang X, Li R, Zhang ZY, Fang J, Zhang X. Effects of Probiotic Preparations on Inflammatory Cytokines in Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis. Curr Pharm Biotechnol. 2021;22(10):1338-1349. doi: 10.2174/1389201021666201119124058. PMID: 33213338.
- Zaidan N, Nazzal L. The Microbiome and Uremic Solutes. Toxins (Basel). 2022 Mar 30;14(4):245. doi: 10.3390/toxins14040245. PMID: 35448854; PMCID: PMC9033124.
- Pei M, Wei L, Hu S, Yang B, Si J, Yang H, Zhai J. Probiotics, prebiotics and synbiotics for chronic kidney disease: protocol for a systematic review and meta-analysis. BMJ Open. 2018 Jul 28;8(7):e020863. doi: 10.1136/bmjopen-2017-020863. PMID: 30056379; PMCID: PMC6067341.
- Kanbay M, Onal EM, Afsar B, Dagel T, Yerlikaya A, Covic A, Vaziri ND. The crosstalk of gut microbiota and chronic kidney disease: role of inflammation, proteinuria, hypertension, and diabetes mellitus. Int Urol Nephrol. 2018 Aug;50(8):1453-1466. doi: 10.1007/s11255-018-1873-2. Epub 2018 May 4. PMID: 29728993.
- Alvarenga L, Cardozo LFMF, Borges NA, Lindholm B, Stenvinkel P, Shiels PG, Fouque D, Mafra D. Can nutritional interventions modulate the activation of the NLRP3 inflammasome in chronic kidney disease? Food Res Int. 2020 Oct;136:109306. doi: 10.1016/j.foodres.2020.109306. Epub 2020 May 16. PMID: 32846516.
- Simeoni M, Citraro ML, Cerantonio A, Deodato F, Provenzano M, Cianfrone P, Capria M, Corrado S, Libri E, Comi A, Pujia A, Abenavoli L, Andreucci M, Cocchi M, Montalcini T, Fuiano G. An open-label, randomized, placebo-controlled study on the effectiveness of a novel probiotics administration protocol (ProbiotiCKD) in patients with mild renal insufficiency (stage 3a of CKD). Eur J Nutr. 2019 Aug;58(5):2145-2156. doi: 10.1007/s00394-018-1785-z. Epub 2018 Aug 3. Erratum in: Eur J Nutr. 2018 Oct 9;: PMID: 30076458; PMCID: PMC6647244.
- Lempert KD. Probiotics and CKD Progression: Are Creatinine-Based Estimates of GFR Applicable? Am J Kidney Dis. 2019 Oct;74(4):429-431. doi: 10.1053/j.ajkd.2019.02.003. Epub 2019 Mar 22. PMID: 30910371.
- Feng Z, Wang T, Dong S, Jiang H, Zhang J, Raza HK, Lei G. Association between gut dysbiosis and chronic kidney disease: a narrative review of the literature. J Int Med Res. 2021 Oct;49(10):3000605211053276. doi: 10.1177/03000605211053276. PMID: 34704483; PMCID: PMC8554569.
- Mafra D, Borges N, Alvarenga L, Esgalhado M, Cardozo L, Lindholm B, Stenvinkel P. Dietary Components That May Influence the Disturbed Gut Microbiota in Chronic Kidney Disease. Nutrients. 2019 Feb 27;11(3):496. doi: 10.3390/nu11030496. PMID: 30818761; PMCID: PMC6471287.
- Koppe L, Soulage CO. Preservation of residual kidney function to reduce non-urea solutes toxicity in haemodialysis. Nephrol Dial Transplant. 2020 May 1;35(5):733-736. doi: 10.1093/ndt/gfz224. PMID: 31711183.
- Gurley A, O'Brien T, Garland JM, Finn A. Lactococcus lactis bacteraemia in a patient on probiotic supplementation therapy. BMJ Case Rep. 2021 Jul 14;14(7):e243915. doi: 10.1136/bcr-2021-243915. PMID: 34261634; PMCID: PMC8280906.
- Patrícia A, Gomes P. A Gut microbiota and recent developments regarding its impact on health and disease. 2017. https://repositorio.ul.pt/bitstream/10451/36100/1/MICF_Ana_Patricia_Gomes.pdf

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
