Archive of Urological Research
True collision renal tumour of oncocytoma and papillary Renal cell carcinoma: Case Report and Review of the Literature
Krzysztof Stempel1*, Ali Bencherki1, Nils Tedehammar1, Erik Sagen2 and Saad Elzanaty3
2Department of Urology, NU Hospital Group, Uddevalla, Sweden
3Department of Translational Medicine, Skåne University Hospital, Lund University, Malmö, Sweden
Cite this as
Stempel K, Bencherki A, Tedehammar N, Sagen E, Elzanaty S (2020) True collision renal tumour of oncocytoma and papillary Renal cell carcinoma: Case Report and Review of the Literature. Arch Urol Res 4(1): 080-084. DOI: 10.17352/aur.000025Background: The clear cell, chromophobe and papillary carcinoma as well as oncocytoma are the most common renal tumours. While cases of hybrid renal tumours are well known, the true collision renal tumour of oncocytoma and Papillary Renal Cell Carcinoma (PRCC) is still rare. Herein, we present a case of a true collision renal tumour of an oncocytoma and a PRCC as well as a review of the literature.
Case presentation: A 53- year- old man presented with painless macroscopic haematuria and nocturia. While urethrocystoscopy showed no pathological findings of the bladder mucosa, a CT scan of the abdomen and thorax revealed a 12x17x16 cm renal mass. The patient underwent a subcostal transperitoneal radical left sided nephrectomy. Pathological examination revealed a true collision tumour of an oncocytoma and a PRCC; type 1, Fuhrman grade 2 (pT1a). No clinical, laboratory or radiological signs of local recurrence or distant metastases were seen during the nine-year follow-up period.
Conclusions: According to our and previous case reports, collision renal tumours consisting of an oncocytoma and a PRCC seems to have a good prognosis and does not require a specific follow-up program but rather can follow the standard program for PRCC.
Introduction
Oncocytoma is the most common benign renal tumour. Its incidence is less common and represents 3 – 7 % of all renal tumours [1]. It originates from the intercalated cells of the collecting duct [2]. It occurs in people of all ages but most commonly in men above 50 [2]. In general, oncocytoma is associated with almost 100 % disease-specific survival rate [3]. Histologically, oncocytoma consists of round-to polygonal-shaped cells with an abundant finely granular cytoplasm [4].
Papillary Renal Cell Carcinoma (PRCC) is the second most common Renal Cell Carcinoma (RCC) comprising 10-15 % of all renal tumours [1]. It occurs in a broad age range similar to other subtypes of RCC from early adulthood to old age, with a peak incidence between the sixth and seventh decades (mean 50–55 years) [5]. The PRCC originates from the proximal tubular epithelial cells [6] and is subdivided into two types; I and II. Type I having an overall better prognosis, outcome, and survival [7]. Histological and immunohistochemical studies of PRCC include cytokeratin 7 (CK7; diffusely positive in the majority of type 1 PRCCs) and Alpha-Methylacyl-CoA Racemase (AMACR) (diffuse granular cytoplasmic positivity) [8].
Renal tumours with hybrid features such as oncocytoma and chromophobe renal cell carcinoma are well documented in the literature [9], whereas collision renal tumours, defined as the coexistent but independent tumours that are histologically distinct [10], such as PRCC and oncocytoma, are very rare.
Herein, we present a case of a true collision renal tumour of an oncocytoma and a PRCC together with a review of the literature.
Case presentation
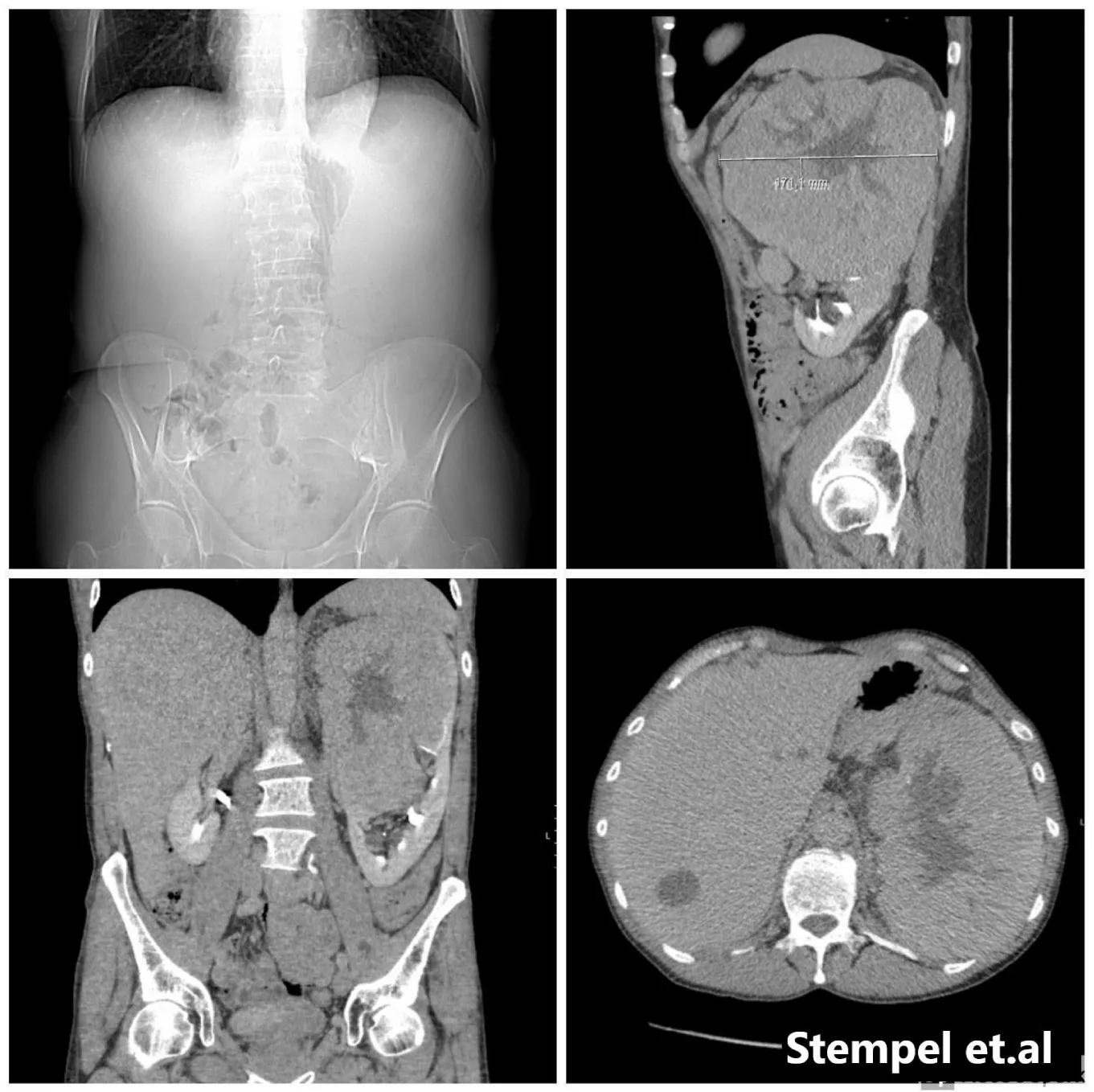
We present a 53-year-old male without significant past medical history and no family history of renal cell carcinoma. As such, no genetic survey was carried out. He presented with painless macroscopic haematuria and nocturia. A digital rectal examination revealed a slight asymmetry of the prostate with no suspicion of malignancy. A urethrocystoscopy showed apart from a somewhat long supracollicular distance and an easily bleeding mucosa of the prostatic urethra, no pathological changes in the bladder´s mucosa. A CT- scan of the abdomen and thorax revealed 12×17×16 cm mass on the superior lobe of the left kidney, with a radiological preliminary diagnosis of a renal tumour with central necrosis. On the right kidney, a 1 cm lesion was described as a nodule of unclear nature. Several aortic and supradiaphragmatic nodes up to 1, 5 cm in diameter were suspected of being metastatic. With no signs of metastases to the lungs (Figure 1).
The patient underwent a subcostal transperitoneal radical left sided nephrectomy. No sign of venous thrombosis was found. The procedure was completed successfully without complications, a 2 kg en-bloc specimen and additional adipose tissue suspected of containing numerous metastatic lymph nodes was sent to pathology for analysis. No adrenelectomy was performed. The patient was discharged after five days of postoperative care with no signs of complications.
The pathology report had initially revealed a mixed type of tumour where the majority consisted of oncocytoma that measured 17 cm with multifocal PRCC. No metastatic lymph nodes were found but only healthy adipose tissue. Re-examination of the pathological slides was done at the department of pathology, Gothenburg University Hospital. This revealed a true collision tumour composed of oncocytoma and papillary renal cell carcinoma, type 1, Fuhrman grade 2 (pT1a tumour + oncocytoma).
Eighteen days postoperatively both PET-CT and scintigraphy showed no metastatic disease. Because of the rarity of this tumour, the recommendation of the multidisciplinary conference was to follow-up the patient initially with a six-month timeframe using CT-scan for signs of local recurrence or metastases. The lesion in the right kidney insignificantly reduced in size and a renal ultrasound with contrast revealed a 1 cm lesion interpreted as angiomyolipoma alternatively oncocytoma with difficulty to rule out malignancy.
Therefore, the case was discussed once again in a multidisciplinary conference that recommended a yearly follow up with a CT thorax and abdomen.
During the 9-year follow–up, the CT examinations have not shown signs of local recurrence or metastases and no change in the size of the lesion in the right kidney.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Discussion
Collision renal tumours have been previously described consisting mainly of oncocytoma and chromophobe renal cell carcinoma. The coexistence of oncocytoma and PRCC is rare. We describe a case of oncocytoma and papillary RCC. Our presentation is in line with previous reports that the possibility of mixed malignant tumours should be considered when treating benign renal tumours [2,11-19].
While our patient presented with painless macroscopic haematuria, other reported symptoms at presentation varied from asymptomatic to abdominal pain and/or isolated macroscopic haematuria (Table 1). Interestingly, one case presented with B-symptoms of malignancy (such as lethargy, anorexia and hypercalcaemia) [15] (Table 1). Painless macroscopic haematuria and flank/abdominal pain are two classic symptoms of renal malignancy. The mentioned B-symptoms of malignancy are suggested to be secondary to hypercalcemia, which was the case in that patient [15]. It has been documented that 13-20% of patients with RCC are associated with hypercalcemia [20]. In other words, symptoms at presentation of renal mixed tumours did not differ from patients with other renal tumours.
With the exception of two cases [18,19] (Table 1), our patient was relatively younger (53-years old) compared to other cases [2,11-17] (Table 1). Overall, the median age of men at diagnosis was 68 years, in accordance with reported median age of other renal tumours [21]. Regarding the gender, it shows tendency toward male predominance with reported 11 (92 %) male cases including our case and one female (8%), in disagreement with sex distributions of known renal tumours [22] (Table 1). However, it is unfair to make a conclusion regarding sex distribution in cases with mixed oncocytoma and PRCC due to the shortage in the number of registered patients.
The diagnosis of collision renal tumour is based on histological and immunohistochemical studies. In fact, our case and previous cases have pathological evidence of coexisting mixed oncocytoma and PRCC (Table 2). The decision of active surveillance versus active treatment is depending mostly on the results of histological studies. The size of oncocytoma varies from 1.5 cm to 17 cm with a median size of 4,7cm. PRCC configuration varies from singular foci varying in size from 0.7 cm to 3.4 cm to multifocal, nest-like configurations with a median size of 1 cm. Previous reports indicating increased risk of recurrence with tumour size above 4 cm [23]. The subtype of PRCC was given in 8/12; being type I in five cases (63%), and type II in 3 cases (37 %). Previous studies did not identify the subtype of PRCC as a strong prognostic factor [24]. The Fuhrman grade was available for 6/12 cases including our own, where five are Fuhrman grade 2 (83 %) and Fuhrman grade 1 in one case (17 %). There is insufficient data regarding the prognostic significance of Fuhrman grading although in two series grading did not achieve a significant relationship with outcome on multivariate analysis that included tumour stage [25,26]. Data on staging was available only in 3/12 cases where all are pTa1 (Table 3). Altogether, mixed oncocytoma and PRCC seem to have overall good prognosis.
A collision tumour contains two distinct tumours of different topographical origins [27], with the oncocytoma being the largest component, most often engulfing the PRCC component, which may be a singular lesion or nest-like/blended [12,18] including our case. Thus, it was suggested that oncocytoma of any larger size may have had small PRCC contained within but were not detected when sectioned prior to paraffin embedding [2]. The exact mechanisms behind collision renal tumours are not known, however, theories such as that the lesions either arise from a common precursor that at a later stage of tumorigenesis differentiate into topographically different tumours or that two unrelated cell lines develop topographically different tumours, either synchronously or metachronously, have been suggested [28].
The use of a predicting prognosis nomogram or a staging system such as the University of California Los Angeles Integrated Staging System or UISS [29] could have been useful and interesting to allow a comparison of more parameters of this type of tumors with existing data from two separate entities (Oncocytoma and PRCC). However, unfortunately none of the authors included these parameters in the cases studied, making it difficult to obtain any form of prognostic value. In our case, after following the UISS staging system, our patient was classified as a *low risk* and has a 91.1% chance for a 5-year survival.
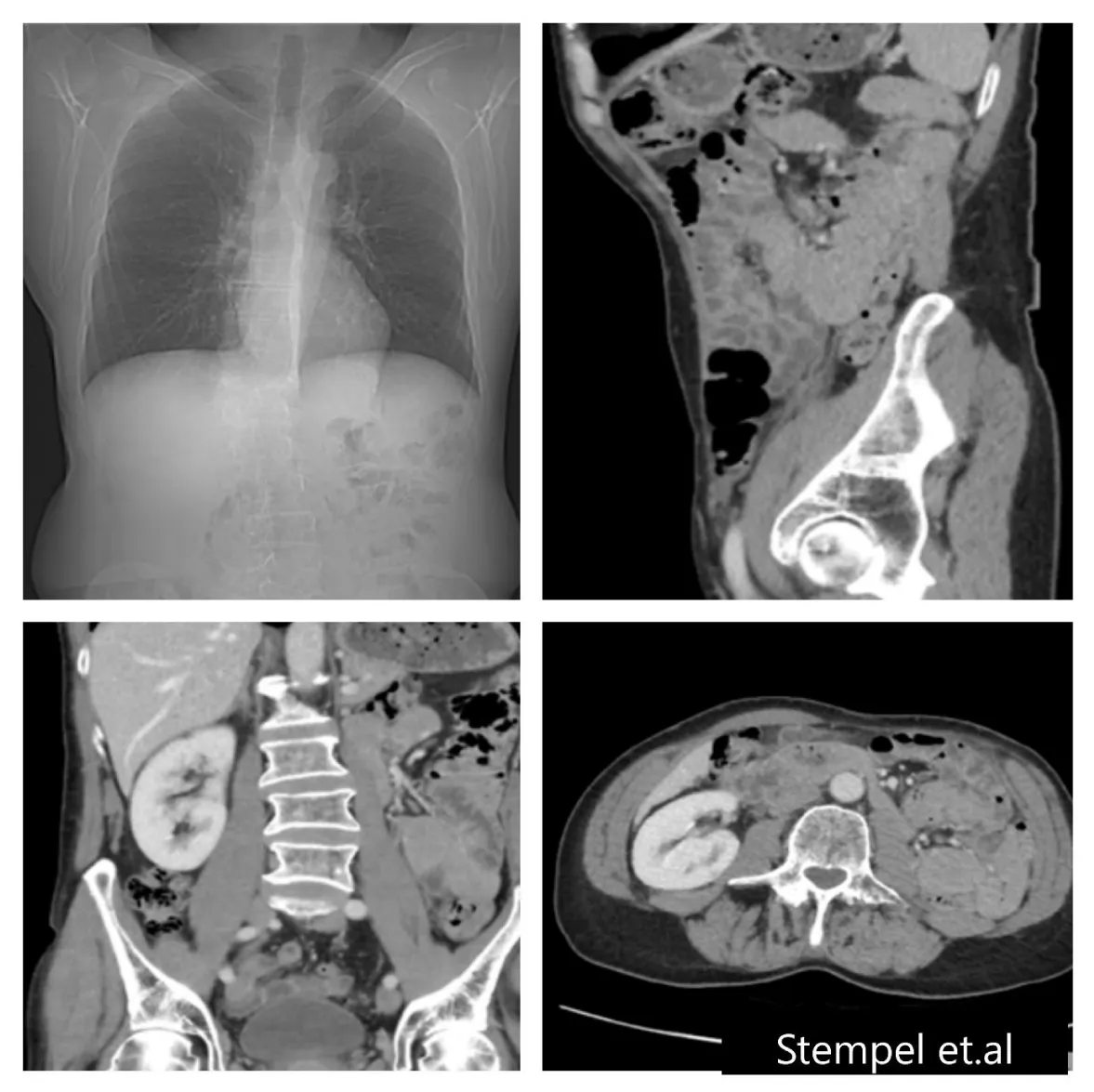
The reported follow–up period in previous cases was up to 5-years. Our case was followed-up for 9-years. The follow up was according to the standard program for PRCC with CT-scanning of the thorax and abdomen twice/year during the first two years then once yearly. No signs of local recurrence or metastases were reported during the 9-years follow-up period (Figure 2). Our results are in accordance with previous reports [11,12,15-17], indicating that no additional follow-up manner is required in such cases with good prognosis of oncocytoma mixed with PRCC.
Conclusion
The possibility of presence of malignant components should be considered when treating benign renal tumours. Renal collision tumour of oncocytoma and PRCC seems to have a good prognosis and doesn’t require a specific follow–up programme rather than the standard program for PRCC.
Authors contributions
All authors should have made substantial contributions to all of the following: the conception and design of the study, or acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be submitted.
- Dechet CB, Bostwick DG, Blute ML, Bryant SC, Zincke H (1999) Renal oncocytoma: multifocality, bilateralism, metachronous tumor development and coexistent renal cell carcinoma. J Urol 162: 40-42. Link: https://bit.ly/2HDpiUt
- McCroskey Z, Sim SJ, Selzman A, Ayala AG, Ro JY (2017) Primary collision tumors of the kidney composed of oncocytoma and papillary renal cell carcinoma: A review. Ann Diagn Pathol 29: 32-36. Link: https://bit.ly/3nVq6DK
- Tomas G, Hardarson S, Petursdottir V, Thoroddsen A, Magnusson J, et al. (2005) Renal Oncocytoma: A Clinicopathological Analysis of 45 Consecutive Cases. BJU Int 96: 1275-1279. Link: https://bit.ly/33je1Ag
- Andaloussi Benatiya M, Rais G, Tahri M, Barki A, El sayegh H, et al. (2012) Renal Oncocytoma: Experience of Clinical Urology A, Urology Department, CHU Ibn Sina, Rabat, Morocco and Literature Review. Pan Afr Med J 12: 24. Link: https://bit.ly/3mc4zq2
- Efrat T, Matvey T, Kae JT, Longo T, Zukerman Z, et al. (2017) Body mass index and the clinicopathological characteristics of clinically localized renal masses—An international retrospective review. Urol Oncol 35: 459.e1-459.e5. Link: https://bit.ly/3pZsLhw
- Rini BI, Campbell SC, Escudier B (2009) Renal cell carcinoma. Lancet 373: 1119-1132. Link: https://bit.ly/39gNp6J
- Sandra S, Martin J, Frederik CR, Becker F, Schumacher S, et al. (2012) Incidence and long-term prognosis of papillary compared to clear cell renal cell carcinoma--a multicentre study. Eur J Cancer 48: 2347-2352. Link: https://bit.ly/33m2Jvk
- American Urological Association (2020) Papillary Renal Cell Carcinoma - American Urological Association. Www.Auanet.Org, 2020,
- Mikami S, Kuroda N, Nagashima Y, Ohe C, Hayashi H, et al. (2019) Classification of Solid Renal Tumor with Oncocytic/Eosinophilic Cytoplasm: Is Hybrid Oncocytic/Chromophobe Renal Tumor a Subtype of Oncocytoma, Chromophobe Renal Cell Carcinoma, or a Distinct Tumor Entity? Ann Transl Med 7: S350. Link: https://bit.ly/377AfGF
- Brandwein-Gensler M, Urken M, Wang B (2004) Collision Tumor of the Thyroid: A Case Report of Metastatic Liposarcoma plus Papillary Thyroid Carcinoma. Head Neck 26: 637-641. Link: https://bit.ly/2JdP4PT
- Goyal R, Parwani AV, Gellert L, Hameed O, Giannico GA (2015) A Collision Tumour of Papillary Renal Cell Carcinoma and Oncocytoma: Case Report and Literature Review. Am J Clin Pathol 144: 811-816. Link: https://bit.ly/37aIBNz
- Al-Saleem T, Balsara BR, Liu Z, Feder M, Testa JR (2005) Renal oncocytoma with loss of chromosomes Y and 1 evolving to papillary carcinoma in connection with gain of chromosome 7. Coincidence or progression? Cancer Genet Cytogenet 163: 81-85. Link: https://bit.ly/3mbVRb9
- Rowsell C, Fleshner N, Marrano P, Squire J, Evans A (2006) Papillary renal cell carcinoma within a renal oncocytoma: case report of an incidental finding of a tumour within a tumour. J Clin Pathol 60: 426-428. Link: https://bit.ly/39geeYN
- Vasuri F, Fellegara G (2008) Collision Renal Tumours. Int J Surg Pathol 17: 338-339. Link: https://bit.ly/37d5IHx
- Floyd MS, Javed S, Pradeep KE, De Bolla AR (2011) Composite Oncocytoma and Papillary Renal Cell Carcinoma of the Kidney Treated by Partial Nephrectomy: A Case Report. Sci World J 11: 1173-1177. Link: https://bit.ly/2JeFxIc
- Sejben I, Szabó Z, Lukács N, Loránd M, Sükösd F, et al. (2013) Papillary renal cell carcinoma embedded in an oncocytoma: Case report of a rare combined tumour of the kidney. Can Urol Assoc J 7: 1911-6470. Link: https://bit.ly/3nXQwou
- Özer C, Gören MR, Egilmez T, Bal N (2014) Papillary renal cell carcinoma within a renal oncocytoma: Case report of very rare coexistence. Can Urol Assoc J 8: 11-12. Link: https://bit.ly/3652CpG
- Baydar DE (2016) Mixed Oncocytoma and Papillary Renal Cell Carcinoma. Ann of Clin Case Rep 1: 1025. Link: https://bit.ly/3q2pQER
- Williamson SR, Cheng L, Gadde R, Giannico GA, Wasco MJ, et al. (2019) Renal cell tumors with an entrapped papillary component: a collision with predilection for oncocytic tumors. Virchows Arch 476: 399-407. Link: https://bit.ly/3l8XbKG
- Palapattu G, Kristo B, Rajfer J (2002) Paraneoplastic Syndromes in Urologic Malignancy: The Many Faces of Renal Cell Carcinoma. Nat Rev Urol 4: 163-170. Link: https://bit.ly/37dKwB0
- Thompson RH, Ordonez MA, Iasonos A, Secin FP, Guillonneau B, et al. (2020) Renal Cell Carcinoma in Young and Old Patients–Is There a Difference? J Urol 180: 1262-1266. Link: https://bit.ly/2JbqEX9
- Mancini M, Righetto M, Baggio G (2020) Gender-Related Approach to Kidney Cancer Management: Moving Forward. Int J Mol Sci 21: 3778. Link: https://bit.ly/39grXyQ
- Gupta G, Adhikary SD, Kumar S, Chacko NK, Kekre NS, et al. (2008) Histopathological Analysis of T1 Renal Cell Carcinoma: Does Presentation Matter? Indian J Urol 24: 504-507. Link: https://bit.ly/3maN8G9
- Ren W, Gao X, Zhang X, Hu J, Li H, et al. (2019) Prognostic Factors for the Survival of Patients with Papillary Renal Cell Carcinoma after Surgical Management. Clin Transl Oncol 22: 725-733. Link: https://bit.ly/33j6SjH
- Ficarra V, Righetti R, Martignoni G, D'Amico A, Pilloni S, et al. (2001) Prognostic Value of Renal Cell Carcinoma Nuclear Grading: Multivariate Analysis of 333 Cases. Urol Int 67: 130-134. Link: https://bit.ly/39iLgYm
- Hyung LK, Amnon Z, Ken-Ryu H, Figlin RA, Belldegrun AS (2004) Prognostic Significance of Venous Thrombus in Renal Cell Carcinoma. Are Renal Vein and Inferior Vena Cava Involvement Different? J Urol 171: 588-591. Link: https://bit.ly/3leMq9O
- Shoaib RT, Farzan R, Neha S, Srivastava S (2014) Collision Tumour of the Palate: A Rare Case Report. Contemp Clin Dent 5: 102-105. Link: https://bit.ly/3fBnSGv
- Burch-Smith R, Tannir NM, Resetkova E, Tamboli P, Rao P (2014) Collision tumour of the kidney composed of clear cell carcinoma and collecting duct carcinoma: report of a case with unusual morphology and clinical follow up. Chin J Cancer 33: 351-355. Link: https://bit.ly/39hsW1N
- Zisman A, Pantuck AJ, Dorey F, Said JW, Shvarts O, et al. (2001) Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol 19: 1649-1657. Link: https://bit.ly/3maMwQR
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
