Archive of Urological Research
Association between Metabolic Syndrome and tumor histologic grade and pathologic stage of bladder cancer
Shan Jiang1,2, Zhi-He Xu1,2, Qin-Feng Xu2, Kun Ding2, Ming-Zhen Yuan2, Yong Guan1,2* and Sheng-Tian Zhao1*
2Department of Urology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan 250021, China
Dr. Yong Guan, Department of Urology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan 250021, China, E-mail: [email protected]
Cite this as
Jiang S, Xu ZH, Xu QF, Guan Y, Zhao ST, et al. (2020) Association between Metabolic Syndrome and tumor histologic grade and pathologic stage of bladder cancer. Arch Urol Res 4(1): 085-089. DOI: 10.17352/aur.000026Background: As we all know,The Metabolic syndrome (MetS) is an emerging condition worldwide, has become an important public health problem. MetS is consistently associated with an increased risk of several cancers.This study aimed to evaluate the association between the MetS and tumor grade and stage of bladder cancer.
Methods: We retrospectively analyzed data of 280 patients who were diagnosed bladder cancer from the Department of Urology, Shandong Provincial Hospital between May 2013 and January 2019. Clinical staging was determined accriding to the 2002 TNM classification. Tis,Ta and T1 tumors were accepted as lower stage and T2,T3 and T4 as higher stage. The histologic grade was determined according to the 2004 World Health Organization grading system. We evaluated the predictive risk factors for the bladder cancer and MetS, including age,sex,BMI,the presence of hypertension, diabetes mellitus, High-Density Lipoprotein (HDL) and triglyceride. Analyses were completed using chi-square tests and logistic regression analysis.
Results: Among the 280 patients in our study,225 were male and 55 were female. MetS was found in 84 patients. 148 patients had hpertension, 114 patients had diabetes mellitus and 129 had a body mass index ≥25 kg/m2. MetS was significantly associated with histologic stage (P=0.021) of bladder cancer.In the binary logistic regression analysis, the presence of MetS predicts the risk of higher T stage(odds ratio=0.204,P=0.001).
Introduction
Bladder cancer is one of the most common malignancy of the urinary system,ranks among the 10 highest incident cancers worldwide. It’s a leading cause of cancer-related deaths [1]. The data which provided by the International Agency for Research on Cancer in 2012 suggested that 55,486 cases and 26,820 deaths were estimated for the population in the People’s Republic of China, accounts for a large proportion of bladder cancer in East Asia (85,451 cases and 37,491 deaths). Several risk factors are directly associated with an increased risk of bladder cancer, such as smocking and occupational exposure [2]. Besides, some studies have reported that obesity is involed in the increased risk of incident bladder cancer [3-5]. Extensive epidemiological evidence suggests that obesity is associated with an increased risk of cancer, while physical activity can reduce the risk of cancer [6], but bladder cancer is not always associated with body size or physical activity [7]. However, little data are available with regard to Body Mass Index (BMI) and the tumor grade and stage of bladder cancer.In epidemiological studies examining obesity and bladder cancer [8-22], no statistically significant association was found in most populations [3,9-11,13,15-18,20-22]. Although three prospective studies [8,12,14] and a large case-control study [19] had found a positive association between obesity and the risk of bladder cancer, only one [14] of these studies examined the association in detail.
As we all know, Metabolic Syndrome (MetS) is a complicated disorder described as a cluster of abnormal metabolic factors related to excess calorie intake and sedentary lifestyles. To our knowledge, MetS is characterized by overweight, blood glucose, hypertension, and dyslipidemia, which has become a global health problem with growing prevalence. With the improvement of living standard, the incidence and prevalence of obesity continues to rise. The Body Mass Index (BMI) is an important international measure of obesity and health. Association beetween BMI and outcomes after surgery has been reported; however ,there is a limited large-sample research to evaluate the relationship between the BMI and grade and stage of malignancy of bladder cancer in a Chinese population. In our study, we investigated the relationship between the grade and stage of malignancy of bladdeer cancer and BMI and Mets in a Chinese population.
Materials and methods
After obtaining approval from the Shandong Provincial Hospital, we retrospectively analyzed the data of 280 patients who were diagnosed bladder cancer from May 2013 to January 2019. Clinical and pathological data were retrospectively obtained from patient electronic medical records, including age, sex, height, weight, Body Mass Index (BMI), blood pressure, diabetes mellitus, high-density lipoprotein (HDL), triglyceride, tumor grade, and stage. Pathologic staging was determined according to the 2002 TNM classification. Tis, Ta and T1 tumors were defined as lower stage and T2, T3 and T4 tumors were defined as higher stage. The histologic grade was determined according to the 2004 World Health Organization grading system.
Statistical analysis was carried out on a personal computer using statistical software (SPSS, version 17.0; SPSS Inc., Chicago, IL, USA). Analyses were completed using chi-square tests and logistic regression analysis. All tests were two-sided with P<0.05 considered to be significant.
MetS was diagnosed when complying with three or more of the following abnormalities:1) BMI≥25.0 kg/m2 , 2) fasting plasma glucose≥6.1 mmol/L or 2-hour plasma Glucose≥7.8mmol/L, 3) systolic blood pressure≥140mmHg or diastolic blood pressure≥90 mmHg, 4) triglyceride (TG)≥1.70 mmol/L or High-Density Lipoprotein Cholesterol (HDL-C)<0.9 mmol/L in males and<1.0 mmol/L in females. BMI was calculated as weight in kilograms divided by the square of height in meters.
Results
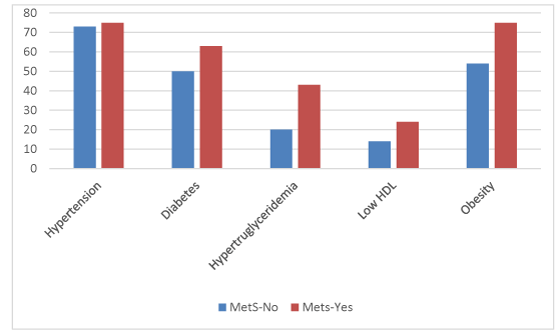
As showned in the Table 1, among the 280 patients who were analyzed in our study, 225 were male and 55 were female, with an average of 69.44±9.158 years. There were 71(25.4%) patients who were defined as higher stage and 209(74.6%) patients were defined as lower stage. MetS was found in 84(30.0%) patients. 148 patients had hpertension, 114 patients had diabetes mellitus and 129 had a body mass index ≥25 kg/m2. Besides, 154(55.0%) patients were defined as higher grade and 126(45.0%) patients were difined as lower grade. Figure 1 displayed the MetS stratified MetS condition distribution. It is obvious that hypertension and obesity are the most frequent MetS components in both groups.
The clinicopathological demographics of characteristics between patients with or without MetS are demonstrated in Table 2. According to our analysis, MetS was significantly associated with pathologic stage (P=0.021) of bladder cancer. Besides, there also had an significant association between MetS and BMI(P<0.001. To some extent, MetS were related to obesity.
The association between MetS (and the components of MetS) and the pathologic stage and histologic grade are depicted at Table 3. In the binary logistic regression analysis, the presence of MetS predicts the risk of higher T stage (odds ratio=0.204,P=0.001). Besides, BMI also has an siginificantly association with pathologic stage(odds ratio=0.785,P<0.001 and histologic grade odds ratio=0.881,P=0.006.
Discussion
In recent years, a number of epidemiological studies have shown that the patients with MetS in a Chinese population were found to have statistically significant with the occurrence and development of urinary tract diseases, and a large number of research confirmed that MetS was significantly associated with the pathogenesis and prognosis of prostate cancer. However, there are few information was available on the association between MetS, as well as the components of MetS, and bladder cancer.
Why is Mets associated with the pathogenesis and prognosis of the bladder cancer? Mets can reduce insulin sensitivity and lead to permanent insulin excess [23]. In addition, our report suggests that high BMI levels may be a risk factor for upper stage of bladder urothelial carcinoma.
The link mechanism between MetS and cancer risk is not fully understood. However, some MetS components have been extensively studied as risk factors for cancer. Increasing evidence shows the negative impact of obesity on genitourinary malignancies [24]. Some epidemiological studies have shown a positive correlation between obesity and increased bladder cancer risk, although other studies have not found any significant associations [7,14].
In our preseant study, we retrospectively analyzed 280 patients undergoing transurethral resection or radical cystectomy surgery of a bladder tumor to assess the relationship between the MetS and the sgressiveness of urothelial carcinoma of the bladder. Besides, we also investigated the association between the components of the MetS(DM, BMI , hypertension, hypertruglyceridemia, low HDL) and bladder cancer histologic grade and stage. We found an significantly association of obesity and bladder cancer including the tumor histologic grade and pathologic stage in a Chinese population.
The biological mechanism of carcinogenesis associated with obesity is unclear, but many possibilities have been proposed.High levels of adipose tissue are related to high levels of cholesterol, which is the precursor of the androgen testosterone, which stimulates the proliferation of epithelial cells.High fat levels are also associated with high plasma levels of vascular endothelial growth factor (VEGF), both of which stimulate the proliferation of epithelial cells.Adipose tissue also secretes leptin, which is related to promoting angiogenesis, so it may also promote tumor development [25].
Obesity is closely related to many major chronic diseases, including coronary heart disease and tumors, and has become a global public health problem. Studies have shown that overweight/obesity is an important cause of diabetes, and weight status in early adulthood and weight changes in middle age can increase the risk of chronic diseases such as diabetes and coronary heart disease. Increased body size and physical inactivity are associated with an increased risk of several cancers, but there are few studies suggested the relationship between Body Mass Index (BMI) and bladder cancer. So, in addition to the analysis of the relationship between MetS and bladder cancer, we researched the association between the BMI≥25.0 which as an individual factor and bladder cancer. As was shown in the Tables 2,3, we can found that BMI was sigificantly associated with bladder cancer including the tumor histologic grade and pathologic stage in a Chinese population. The positive correlation between BMI and bladder cancer was independent of other known bladder cancer risk factors, including age, race, and smoking, implying that avoiding obesity may play an important role in bladder cancer prevention.
Obesity exerts an influence on the condition of the metabolic which it can cause downstream metabolic changes, such as inflammation, higher insulin levels, insulin-like growth factor production, and changes in sex hormones, all of these factors are related to cancer [26,27]. However, obesity and MetS are complex problems in the elderly. With the growth of age, the body will have some changes, such as the decrease of height, the decrease of muscle, the increase of fat in insulin resistance area, etc., but BMI is often not so accurate.(such as visceral, subcutaneous, intramuscular, and intrahepatic fat) [28]. With age, sarcopenia or sarcopenia is particularly important as it results in insulin resistance and MetS.
On the contrary, older adults are subject to the“Obesity Paradox” in which certain outcomes,such as survival,compared with normal weight or morbidly obese patients, obese patients have better curative effects.Research on other malignancies has shown that there is an Obesity Paradox, despite limited data specific to older people [29-30].
Conclusion
Our research implying that the MetS and obseity had associstion with the bladder caner, not only the tumor pathologic stage but also the histologic grade. In other words, when the patients are considered MetSs, they were found to have statistically significant higher T stage and histologic grade of bladder cancer.
Our research implying that the MetS and obseity had associstion with the bladder caner, not only the tumor pathologic stage but also the histologic grade. In other words, when the patients are considered MetSs, they were found to have statistically significant higher T stage and histologic grade of bladder cancer.
Author contributions
STZ and YG designed this study; SJ performed the statistical analysis and drafted the manuscript; and QFX, KD, ZHX, and YG, collected the data and helped design the manuscript. All authors read and approved the final manuscript.
This work was supported by the National Nature Science Foundation of China (No. 81670625 and No. 81470969).
- Murta-Nascimento C, Schmitz-Dräger BJ, Zeegers MP, Steineck G, Kogevinas M, et al. (2007) Epidemiology of urinary bladder cancer: from tumor development to patient's death. World J Urol 25: 285-295. Link: https://bit.ly/3rzGWuD
- Wyszynski A, Tanyos SA, Rees JR, Marsit CJ, Kelsey KT, et al. (2014) Body mass and smoking are modifiable risk factors for recurrent bladder cancer. Cancer 120: 408-414. Link: https://bit.ly/3nXGaoT
- Qin Q, Xu X, Wang X, Zheng XY (2013) Obesity and risk of bladder cancer: a meta-analysis of cohort studies. Asian Pacific journal of cancer prevention. Asian Pac J Cancer Prev 14: 3117-3121. Link: https://bit.ly/3nOWwQC
- Stocks T, Bjørge T, Ulmer H, Manjer J, Häggström C, et al. (2015) Metabolic risk score and cancer risk: pooled analysis of seven cohorts. Int J Epidemiol 44: 1353-1363. Link: https://bit.ly/3hmuCsG
- Cantiello F, Cicione A, Salonia A, Autorino R, De Nunzio C, et al. (2015) Association between metabolic syndrome, obesity, diabetes mellitus and oncological outcomes of bladder cancer: a systematic review. Int J Urol 22: 22-32. Link: https://bit.ly/3aLJsYt
- World Health Organization (2002) International Agency for Research on Cancer. IARC Handbooks of cancer prevention. Volume 6:weight control and physical activity A.
- Koebnick C, Michaud D, Moore SC, Park Y, Hollenbeck A, et al. (2008) Body mass index, physical activity, and bladder cancer in a large prospective study. Cancer Epidemiology, Biomarkers & Prevention 17: 1214-1221. Link: https://bit.ly/3nTt6kr
- Samanic C, Gridley G, Chow WH, Lubin J, Hoover RN, et al. (2004) Obesity and cancer risk among white and black United States veterans. Cancer Causes Control 15: 35-43. Link: https://bit.ly/2WMce36
- Møller H, Mellemgaard A, Lindvig K, Olsen JH (1994) Obesity and cancer risk: a Danish record-linkage study. Eur J Cancer 30a: 344-350. Link: https://bit.ly/2WQFdTy
- Wolk A, Gridley G, Svensson M, Nyrén O, McLaughlin JK, et al. (2001) A prospective study of obesity and cancer risk (Sweden). Cancer Causes Control 12: 13-21. Link: https://bit.ly/2KXhhLp
- Whittemore AS, Paffenbarger RS, Anderson K, Lee JE (1984) Early precursors of urogenital cancers in former college men. J Urol 132: 1256-1261. Link: https://bit.ly/3psW1fz
- Batty GD, Shipley MJ, Jarrett RJ, Breeze E, Marmot MG, et al. (2005) Obesity and overweight in relation to organ-specific cancer mortality in London (UK): findings from the original Whitehall study. Int J Obes (Lond) 29: 1267-1274. Link: https://bit.ly/3rwHdhN
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 348: 1625-1638. Link: https://bit.ly/2Mga6ih
- Holick CN, Giovannucci EL, Stampfer MJ, Michaud DS (2007) Prospective study of body mass index, height, physical activity and incidence of bladder cancer in US men and women. Int J Cancer 120: 140-146. Link: https://bit.ly/3rvP1QX
- Lew EA, Garfinkel L (1979) Variations in mortality by weight among 750,000 men and women. J Chronic Dis 32: 563-576. Link: https://bit.ly/38CBsX7
- Rapp K, Schroeder J, Klenk J, Stoehr S, Ulmer H, et al. (2005) Obesity and incidence of cancer: a large cohort study of over 145,000 adults in Austria. Br J Cancer 93: 1062-1067. Link: https://bit.ly/3rwHott
- Tripathi A, Folsom AR, Anderson KE (2002) Risk factors for urinary bladder carcinoma in postmenopausal women. The Iowa Women's Health Study. Cancer 95: 2316-2323. Link: https://bit.ly/3nOX7lk
- Cantwell MM, Lacey JV, Schairer C, Schatzkin A, Michaud DS (2006) Reproductive factors, exogenous hormone use and bladder cancer risk in a prospective study. Int J Cancer 119: 2398-24401. Link: https://bit.ly/37Rbpfz
- Pan SY, Johnson KC, Ugnat AM, Wen SW, Mao Y (2004) Association of obesity and cancer risk in Canada. Am J Epidemiol 159: 259-268. Link: https://bit.ly/3hmv7D4
- Pelucchi C, La Vecchia C, Negri E, Dal Maso L, Franceschi S (2002) Smoking and other risk factors for bladder cancer in women. Prev Med 35: 114-120. Link: https://bit.ly/38IPK8E
- Harris RE, Chen-Backlund JY, Wynder EL (1990) Cancer of the urinary bladder in blacks and whites. A case-control study. Cancer 66: 2673-2680. Link: https://bit.ly/2M3FX5o
- Vena JE, Graham S, Freudenheim J, Marshall J, Zielezny M, et al. (1992) Diet in the epidemiology of bladder cancer in western New York. Nutr Cancer 18: 255-264. Link: https://bit.ly/34J09Ab
- Serel TA, Turan T, Soyupek S, Aybek Z, Perk H (2003) Urine and serum free IGF-1 levels in patients with bladder cancer: a brief report. Urol Res 31: 297-299. Link: https://bit.ly/3nTN4vb
- Janssen I, Katzmarzyk PT, Ross R (2004) Waist circumference and not body mass index explains obesity-related health risk. The Am J Clin Nutr 79: 379-384. Link: https://bit.ly/37QiRYC
- Cirillo D, Rachiglio AM, la Montagna R, Giordano A, Normanno N (2008) Leptin signaling in breast cancer: an overview. J Cell Biochem 105: 956-964. Link: https://bit.ly/38A4C9o
- Renehan AG, Zwahlen M, Egger M (2015) Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer 15: 484-498. Link: https://bit.ly/3hmHnDD
- Steele CB, Thomas CC, Henley SJ, Massetti GM, Galuska DA, et al. (2017) Vital Signs: Trends in Incidence of Cancers Associated with Overweight and Obesity - United States, 2005-2014. MMWR 66: 1052-1058. Link: https://bit.ly/3aMJRtK
- Cetin DC, Nasr G (2014) Obesity in the elderly: more complicated than you think. Cleve Clin J Med 81: 51-61. Link: https://bit.ly/3rAYuGO
- Hakimi AA, Furberg H, Zabor EC, Jacobsen A, Schultz N, et al. (2013) An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. J Natl Cancer Inst 105: 1862-1870. Link: https://bit.ly/3pkTUue
- McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, et al. (2018) Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. The Lancet Oncol 19: 310-322. Link: https://bit.ly/34QgcMq
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
