Archive of Urological Research
Comparison of Gleason score of Prostate Cancer at Ultrasound/MRI Fusion Target Biopsy vs. Systematic Transrectal Ultrasound Guided Biopsy with Final Gleason score at Radical Prostatectomy
Jinxing Yu1*, Ugo Falagario2,3, Sarah G Winks1, Kendal Angell4, Ann S Fulcher1, Mary A Turner1, Sterling Jones4, Rohan Kankaria4 and Steven C Smith5
2Department of Urology, Virginia Commonwealth University Health System, 401 North 12th Street, Richmond, VA 23298, USA
3Department of Urology and Organ Transplantation, University of Foggia, Foggia, Italy
4School of Medicine, Virginia Commonwealth University Health System, Main Hospital, 401 North 12th Street, Richmond, VA 23298, USA
5Department of pathology, Virginia commonwealth University Health System, USA
Cite this as
Yu J, Falagario U, Winks SG, Angell K, Fulcher AS, et al. (2022) Comparison of Gleason score of Prostate Cancer at Ultrasound/MRI Fusion Target Biopsy vs. Systematic Transrectal Ultrasound Guided Biopsy with Final Gleason score at Radical Prostatectomy. Arch Urol Res 6(2): 017-021. DOI: 10.17352/aur.000039Copyright
© 2022 Yu J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Purpose: To compare accuracy in predicting final Gleason Grade Group (GGG) of Prostate Cancer (PCa) of US/MRI fusion guided target (fBx) vs. systematic Transrectal Ultrasound-Guided (TRUS) biopsy, using histopathologic analysis at prostatectomy as the gold standard.
Materials and methods: After obtaining IRB approval, we retrospectively reviewed records of patients who underwent Radical Prostatectomy (RP) from January 2014 through May 2019 with prior US/MRI fusion guided target or TRUS biopsy. The rates of upgrading (RP GGG > BX GGG), downgrading (RP GGG < BX GGG), and concordance (RP GGG = BX GGG) were compared between the fBx and TRUS groups. Age, PSA, PSA density, and prostate volume were also noted for all patients. Statistical analysis was utilized to assess the data.
Results: A total of 348 men with complete clinical data were included in this study. The rate of downgrading and upgrading in the fBx group was less than in the TRUS biopsy group (14% vs. 19.6%, and 13.2% vs. 19.6%, respectively). The concordance rate was higher in the US-MR fBx group (72.9% vs. 60.7%, p < 0.05)) across all GGG. Notably, lower rates of concordance were found for GGG 1 (24.1%) and GGG 4 (3.6%) in the TRUS Bx group. Patients who underwent US-MR fBx had higher average PSA (9.4 vs. 6.5 ng/ml), higher PSA density (0.3 vs. 0.2 ng/ml2), and lower prostate volume (31 vs. 42 cc). Additionally, biopsy results showed a lower rate of GGG 1 (3.1% vs. 13.2%) and a higher rate of GGG 5 (14.7% vs. 5.5%) in the US-MR fBx group.
Conclusions: Target biopsy has a higher GGG concordance compared to TRUS biopsy (72.9% vs. 60.7%, p < 0.05). In addition, there was less downgrading or upgrading of final PCa GGG in the fBx groups compared to TRUS Bx (14% vs. 19.6%, 13.2% vs. 19.6%, respectively). This finding may have important implications for treatment decisions.
Introduction
An elevated PSA value often causes concern for prostate malignancy and typically requires a biopsy for further evaluation [1,2]. After histopathologic analysis, these biopsy samples are then assigned a Gleason Grade Group (GGG), which will rate the aggressiveness of the disease and help to dictate the patient’s course of treatment [3,4].
Traditionally, there have been several ways to perform prostate biopsies in individuals with elevated PSA values and concern for malignancy [5,6]. Traditionally, most patients underwent systematic Transrectal Ultrasound biopsy (TRUS Bx), with representative samples taken throughout the prostate in a systematic manner [2,5]. Another method, however, that has gained recent popularity and is quickly becoming the method of choice in many institutions is Transrectal US-MR fusion-guided targeted biopsy (US-MR fBx) [7,8]. A multiparametric MRI (mpMRI) of the prostate is obtained prior to the biopsy, allowing for the identification of specific “targets,” areas suspicious for Prostate Cancer (PCa). These targets are marked on the images prior to the biopsy. At the time of biopsy, these images are then fused with those from real-time transrectal ultrasound, providing a guide for sampling the areas suspicious of malignancy [9-11]. Standard systematic samples can also be obtained in the same setting in order to obtain what is believed to be a very comprehensive evaluation of the prostate [7,8].
While the accuracy of detecting clinically significant PCa using US-MR fBx has shown to be superior to non-fusion methods [5,12-14], some questions remain as to the accuracy and clinical utilization in diagnosing the severity of the disease [5,10,15]. Prior studies show that US-MR fBx identifies a higher GGG PCa (higher risk) than systematic TRUS Bx alone [6,15]. Our concern is that the US-MR fBx may overestimate the GGG for the entire tumor and provide a poor representation of the most prevalent grade of underlying cancer. This higher score could lead to more aggressive treatment than needed, with potential increased treatment side effects or complications, such as impotence or urinary incontinence, among others [16-18].
With the increased utilization of fBx [7,8], our study aims to answer the question of whether US-MR fusion-guided target biopsies provide a representative versus unrepresentative high GGG of prostate cancer compared to systematic TRUS biopsy alone.
Subjects and methods
Study population
The University Institutional Review Board (IRB) approved the retrospective study. From January 2014 through May 2019, the records of 348 patients who had undergone radical prostatectomy were reviewed. Prior to the prostatectomy, these patients had received either systematic TRUS biopsies (n = 219) or US-MR fBx (n = 129).
Imaging & biopsy protocol
Every patient who underwent fBx at our institution had a prebiopsy multi-parametric MRI (mpMRI) of the prostate. Multiparametric MRI included T2-weighted, dynamic contrast-enhanced, and diffusion-weighted imaging at 3T or 1.5T with an endorectal coil. Lesions were graded based on the Prostate Imaging Reporting and Data System (PI-RADS) v2 by two experienced genitourinary radiologists. All patients who had a lesion with PI-RADS 3 or higher were offered US/MRI fusion guided biopsy (UroNav device, Invivo, Philip). All patients who received fBx had a 12-core standard template, in addition to multiple, typically 2-4 samples from each target lesion.
In the TRUS Bx group, 12 core samples were biopsied for each patient in a systematic fashion, with samples from the bilateral apex, mid gland, and base, medially and laterally.
In the biopsy specimen, the Gleason score was assigned for each systematic sample as well as each target, and the highest Gleason score determined the grade group. In some cases, multiple targets of the prostate were detected and biopsied, in which cases, the highest GS of the target was used to determine the grade group. Biopsy and surgical specimens were evaluated by experienced GU pathologists, with expertise in the pathology of the prostate.
Pathology specimen processing
After prostatectomy, the surgical margins of the prostate specimen were examined for histological evidence of cancer during the frozen section. After separating the seminal vesicles, the entire gland was serially sectioned into approximately 3mm slices. Alternate slices were submitted for histopathologic examination. Tissue sections of 4 μm - 6 μm thickness were made from each paraffin block, stained with hematoxylin and eosin, and reviewed. The slides were evaluated microscopically for tumor, and the Gleason score was determined with the sum of the two most predominant patterns (primary and secondary) in terms of surface area. The grade group of the tumor was then determined. For a radical prostatectomy specimen, the highest Gleason grade observed was considered the final grade.
Outcome definition and statistical analysis
The primary objective of the study was to evaluate the concordance between bx and prostatectomy GGG in a population of patients who received either US-MR fBx or TRUS Bx. Concordance was defined as biopsy GGG the same as the final GGG from the surgical specimen. Upgrading or downgrading was defined as any increase or decrease in the Grade group or any change in the order of primary grade and second grade when the biopsy GGG was compared to the final GGG based on surgical histopathological analysis. First descriptive statistics were performed. Continuous variables are reported as medians and Interquartile ranges and were tested by the Kruskal-Wallis test. Categorical variables are shown as frequency and proportion and were tested by the chi-square test. The rates of upgrading (RP GGG > BX GGG), downgrading (RP GGG < BX GGG), and the concordance rate (RP GGG = BX GGG) were compared between the two groups.
Statistical analyses were performed using Stata 14 (StataCorp LP, College Station, TX, USA). All tests were 2-sided with a significance level set at p < 0.05.
Results
A total of 348 men with complete clinical data were included in this study. Descriptive characteristics of the population of the two groups are shown in Table 1. Patients who underwent US-MR fBx had a higher PSA (9.4 vs. 6.5 ng/ml, p < 0.0001), higher PSA density (0.3 vs. 0.2 ng/ml2, p < 0.0001), and a lower prostate volume (31 vs. 42 cc, p < 0.0001).
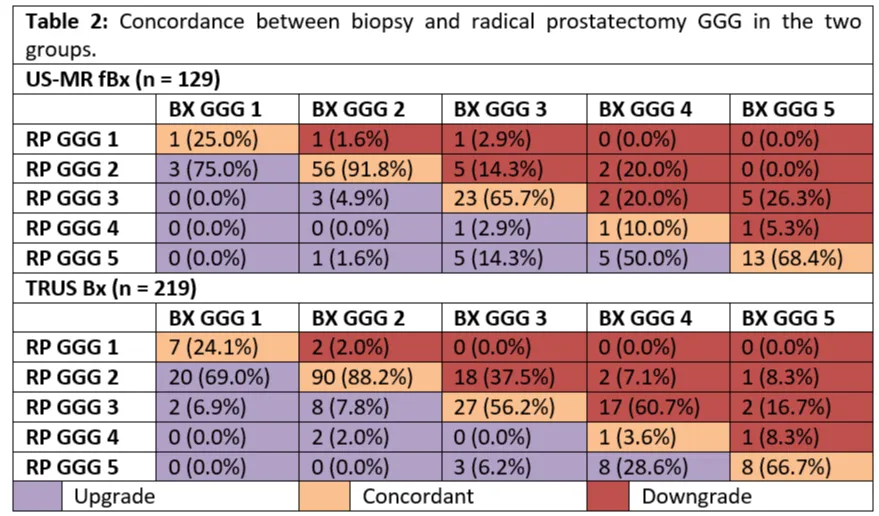
The concordance rate with final GGG was higher in the US-MR fBx group as compared to the TRUS Bx group (94 out of 129, 72.9% vs. 133 out of 219, 60.71%, p < 0.05). Upgrading from Bx to RP was less common in patients who underwent fBx (17 out of 129, 14% vs. 43 out of 219, 19.6%). Downgrading from Bx to RP was also less common in patients who underwent fBx (18 out of 129, 13.2% vs. 43 out of 219, 19.6%). Between both groups, these differences in upgrading and downgrading did not reach statistical significance.
Table 2 shows concordance rates between Bx GGG and RP GGG in the two groups. US-MRI fBx patients had a higher concordance rate across all GGG. The biopsy results showed a similar lower rate of concordance of GGG 1 (25% vs. 24.1%) and GGG 4 (10% vs. 3.6%) in the US-MR fBx group vs the TRUS Bx group, as compared to the GGG 2, 3 and 5.
Discussion
The adoption of US-MR fBx in recent years has led to improved detection of clinically significant PCa [5,12-14]. However, there is a concern that, in the US-MR fBx, the target biopsy specimen obtained is from the most suspicious area within a lesion seen at MRI, which likely represents an area of the lesion with the highest Gleason score. Thus, there is concern that this method of biopsy may result in a downgrading of the PCa at the time of RP and potentially lead to treatment that is more aggressive than necessary [16-18].
In our study comparing the GGG of a standard non-fusion TRUS bx to that of the US-MR fBx, we looked specifically at the rates of GGG upgrading (RP GGG > BX GGG), downgrading (RP GGG < BX GGG), and concordance (RP GGG = BX GGG). We had anticipated seeing the US-MR fBx downgrading rate higher than that of the TRUS Bx, based on previous assumptions and data [6]. We were surprised to see, however, that the GGG of the US-MR fBx in fact had a lower downgrading rate than that of the TRUS Bx, 14% vs. 19.6% (Table 1).
The reasoning for this may have to do with the way core samples are collected and evaluated in each biopsy technique. In the TRUS Bx, generally, 12 cores are taken from different regions of the prostate and each is analyzed individually [19,20]. In our US-MR fBx method, multiple cores are taken from a single target (average of 4.0) in different areas of the tumor itself. These are then provided together as one sample for histopathologic analysis. In this way, if there are both high-grade cores and low-grade cores from the same target, they may average out to a score more representative of the final overall post-prostatectomy GGG. Conversely, in the TRUS Bx, there may only be one core passing through the PCa, and if it passes through an area with the highest malignancy, then it will present a high-grade score for the entire tumor with no other scoring from that area to average against.
We also found it interesting that the US-MR fBx provided a lower rate of upgrading than that of the TRUS Bx, 13.2% vs. 19.6% (Table 1). This may be for the same reason as that of the downgrading. When the urologist or radiologist performing the prostate biopsy has the guidance of the MRI-US fusion images, they may have greater accuracy in sampling the suspicious areas, particularly when taking samples from the different parts of the suspicious area (average 4.0). In other words, the suspicious area might be better represented by fBx with multiple passes through the different parts of the area, as compared to the TRUS Bx with only one pass through a potential lesion. Whatever the reasoning may be, the GGG from TRUS Bx in general has historically proven to be suboptimal in comparison with final pathology [21,22], a finding which was supported by our results. Consequently, the concordance rate was higher in the US-MR fBx group than the TRUS Bx group (72.9% vs. 60.7%, p < 0.05)) across all GGG (Tables 1,2).
Another interesting finding was a difference in the characteristic of prostate size between the TRUS Bx group and the US-MR fBx group (Table 1). The prostate sizes in the TRUS Bx group were on average, about 11 ml larger than those of the US-MR fBx group. One explanation for this may have to do with the fBx group having more patients with intermediate and high GGG (49.6%) as compared to the TRUS Bx group (40.02%) (Table 1). We observed that in many patients who had localized intermediate and high-grade PCa, the prostate sizes were smaller than in the normal population. This explanation, however, would require a more dedicated investigation which has been ongoing in our institution.
The weakness of our study lies in the retrospective design and its inherent selection bias. As our cohort is derived from a large tertiary referral center, some of our patients underwent TRUS bx at outside institutions. This can create a bias in the selection criteria, as biopsies are likely done using different US and biopsy systems, and the experience and skill of the Urologists performing the biopsy vary between institutions. This limitation may be unavoidable in every retrospective analysis. Therefore, a prospective, single-institution design with larger sample size is needed to validate our results.
Conclusion
In conclusion, our data has shown that US-MR fBx has a higher GGG concordance with final pathology from RP compared to TRUS biopsy (72.9% vs. 60.7%, p < 0.05). This may have clinically important implications in choosing treatment options for patients with PCa. In addition, downgrading and upgrading of final PCa GGG by target biopsy are also less frequent compared to TRUS Bx (14% vs. 19.6%, 13.2% vs. 19.6%, respectively), further supporting the impression that US-MR fBx presents a significant advantage over TRUS Bx, providing a GGG that is more accurate and representative of the most dominant underlying PCa.
- Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987 Oct 8;317(15):909-16. doi: 10.1056/NEJM198710083171501. PMID: 2442609.
- Ismail MT, Gomella LG. Transrectal prostate biopsy. Urol Clin North Am. 2013 Nov;40(4):457-72. doi: 10.1016/j.ucl.2013.07.012. Epub 2013 Sep 11. PMID: 24182969.
- Egevad L, Granfors T, Karlberg L, Bergh A, Stattin P. Prognostic value of the Gleason score in prostate cancer. BJU Int. 2002 Apr;89(6):538-42. doi: 10.1046/j.1464-410x.2002.02669.x. PMID: 11942960.
- Montironi R, Santoni M, Mazzucchelli R, Burattini L, Berardi R, Galosi AB, Cheng L, Lopez-Beltran A, Briganti A, Montorsi F, Scarpelli M. Prostate cancer: from Gleason scoring to prognostic grade grouping. Expert Rev Anticancer Ther. 2016;16(4):433-40. doi: 10.1586/14737140.2016.1160780. PMID: 27008205.
- Hara R, Jo Y, Fujii T, Kondo N, Yokoyoma T, Miyaji Y, Nagai A. Optimal approach for prostate cancer detection as initial biopsy: prospective randomized study comparing transperineal versus transrectal systematic 12-core biopsy. Urology. 2008 Feb;71(2):191-5. doi: 10.1016/j.urology.2007.09.029. PMID: 18308081.
- Djavan B, Rocco B. Optimizing prostate biopsy. 2012; BMJ. 344:d8201.
- Verma S, Choyke PL, Eberhardt SC, Oto A, Tempany CM, Turkbey B, Rosenkrantz AB. The Current State of MR Imaging-targeted Biopsy Techniques for Detection of Prostate Cancer. Radiology. 2017 Nov;285(2):343-356. doi: 10.1148/radiol.2017161684. PMID: 29045233; PMCID: PMC5673043.
- Wegelin O, van Melick HHE, Hooft L, Bosch JLHR, Reitsma HB, Barentsz JO, Somford DM. Comparing Three Different Techniques for Magnetic Resonance Imaging-targeted Prostate Biopsies: A Systematic Review of In-bore versus Magnetic Resonance Imaging-transrectal Ultrasound fusion versus Cognitive Registration. Is There a Preferred Technique? Eur Urol. 2017 Apr;71(4):517-531. doi: 10.1016/j.eururo.2016.07.041. Epub 2016 Aug 25. PMID: 27568655.
- Kaplan I, Oldenburg NE, Meskell P, Blake M, Church P, Holupka EJ. Real time MRI-ultrasound image guided stereotactic prostate biopsy. Magn Reson Imaging. 2002 Apr;20(3):295-9. doi: 10.1016/s0730-725x(02)00490-3. PMID: 12117612.
- Pinto PA, Chung PH, Rastinehad AR, Baccala AA Jr, Kruecker J, Benjamin CJ, Xu S, Yan P, Kadoury S, Chua C, Locklin JK, Turkbey B, Shih JH, Gates SP, Buckner C, Bratslavsky G, Linehan WM, Glossop ND, Choyke PL, Wood BJ. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011 Oct;186(4):1281-5. doi: 10.1016/j.juro.2011.05.078. Epub 2011 Aug 17. PMID: 21849184; PMCID: PMC3193933.
- Sonn GA, Natarajan S, Margolis DJ, MacAiran M, Lieu P, Huang J, Dorey FJ, Marks LS. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013 Jan;189(1):86-91. doi: 10.1016/j.juro.2012.08.095. Epub 2012 Nov 14. PMID: 23158413; PMCID: PMC3561472.
- Oberlin DT, Casalino DD, Miller FH, Matulewicz RS, Perry KT, Nadler RB, Kundu S, Catalona WJ, Meeks JJ. Diagnostic Value of Guided Biopsies: Fusion and Cognitive-registration Magnetic Resonance Imaging Versus Conventional Ultrasound Biopsy of the Prostate. Urology. 2016 Jun;92:75-9. doi: 10.1016/j.urology.2016.02.041. Epub 2016 Mar 7. PMID: 26966043; PMCID: PMC4882086.
- Kardos SV, Pan S, Nawaf CB. MP17-08 Mri-Fusion Prostate Biopsy in First-Time Biopsy Cohort Yields Increased Detection of Clinically Significant Prostate Cancer Using A Simplified Mri Grading Scale, 2015. The Journal of Urology, 193(4), p.e179.
- Anastasiadis AG, Lichy MP, Nagele U, Kuczyk MA, Merseburger AS, Hennenlotter J, Corvin S, Sievert KD, Claussen CD, Stenzl A, Schlemmer HP. MRI-guided biopsy of the prostate increases diagnostic performance in men with elevated or increasing PSA levels after previous negative TRUS biopsies. Eur Urol. 2006 Oct;50(4):738-48; discussion 748-9. doi: 10.1016/j.eururo.2006.03.007. Epub 2006 Mar 24. PMID: 16630688.
- Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, Okoro C, Raskolnikov D, Parnes HL, Linehan WM, Merino MJ, Simon RM, Choyke PL, Wood BJ, Pinto PA. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015 Jan 27;313(4):390-7. doi: 10.1001/jama.2014.17942. PMID: 25626035; PMCID: PMC4572575.
- Stanford JL, Feng Z, Hamilton AS, Gilliland FD, Stephenson RA, Eley JW, Albertsen PC, Harlan LC, Potosky AL. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000 Jan 19;283(3):354-60. doi: 10.1001/jama.283.3.354. PMID: 10647798.
- Parker C. Active surveillance: towards a new paradigm in the management of early prostate cancer. Lancet Oncol. 2004 Feb;5(2):101-6. doi: 10.1016/S1470-2045(04)01384-1. PMID: 14761814.
- van den Bergh RC, Roemeling S, Roobol MJ, Aus G, Hugosson J, Rannikko AS, Tammela TL, Bangma CH, Schröder FH. Gleason score 7 screen-detected prostate cancers initially managed expectantly: outcomes in 50 men. BJU Int. 2009 Jun;103(11):1472-7. doi: 10.1111/j.1464-410X.2008.08281.x. Epub 2009 Jan 19. PMID: 19154509.
- Presti JC Jr, O'Dowd GJ, Miller MC, Mattu R, Veltri RW. Extended peripheral zone biopsy schemes increase cancer detection rates and minimize variance in prostate specific antigen and age related cancer rates: results of a community multi-practice study. J Urol. 2003 Jan;169(1):125-9. doi: 10.1097/01.ju.0000036482.46710.7e. PMID: 12478119.
- Siu W, Dunn RL, Shah RB, Wei JT. Use of extended pattern technique for initial prostate biopsy. J Urol. 2005 Aug;174(2):505-9. doi: 10.1097/01.ju.0000165385.53652.7a. PMID: 16006881.
- Hambrock T, Hoeks C, Hulsbergen-van de Kaa C, Scheenen T, Fütterer J, Bouwense S, van Oort I, Schröder F, Huisman H, Barentsz J. Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. Eur Urol. 2012 Jan;61(1):177-84. doi: 10.1016/j.eururo.2011.08.042. Epub 2011 Aug 27. PMID: 21924545.
- Lattouf JB, Saad F. Gleason score on biopsy: is it reliable for predicting the final grade on pathology? BJU Int. 2002 Nov;90(7):694-8; discussion 698-9. doi: 10.1046/j.1464-410x.2002.02990.x. PMID: 12410749.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
