Archive of Urological Research
Molecular investigation of effects of probiotic on papG and iutA genes in different strains of E.coli isolated from urinary tract infection
Romina Rezaei1, Mona Mohammad Aliha1, Roudabeh Behzadi Andouhjerdi2* and Maryam Tajabadi Ebrahimi3
2Department of Genetics, Central Tehran Branch, Islamic Azad University, Tehran, Iran
3Department of Microbiology, Central Tehran Branch, Islamic Azad University, Tehran, Iran
Cite this as
Rezaei R, Aliha MM, Andouhjerdi RB, Ebrahimi MT (2023) Molecular investigation of effects of probiotic on papG and iutA genes in different strains of E.coli isolated from urinary tract infection. Arch Urol Res 7(2): 017-025. DOI: 10.17352/aur.000045Copyright
© 2023 Rezaei R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Escherichia coli-induced urinary tract infections (E.coli-UTIs) are considered one of the most prevalent infections in Iran. One of the important factors of pathogenicity, particularly in its binding to epithelial cells, is the presence of iutA and papG virulence genes in Escherichia coli (E.coli). Lactobacilli, as a group of probiotics, play a vital role in the body and are useful in some cases due to their therapeutic effects. The aim of this study was to evaluate how the molecular activity of Lactobacillus casei PTCC1608 influences different strains of pathogenic E.coli isolated from urinary infections through PCR. In this study, the pathogenic strain of E.coli was isolated from the patients with Urinary Tract Infections (UTIs) and analyzed by the use of the PCR method. Then, the strains with positive genotypes were isolated and the antimicrobial effect of Lactobacillus casei was evaluated through the disc diffusion and dilution methods in liquid medium. The antimicrobial effect of Lactobacillus casei on E.coli bacteria isolated from urine samples from 40 patients with UTI was studied. In addition, the results of the antibiotic sensitivity test at 1:10 dilution showed a growth inhibition zone of 9 mm. The results indicated that the isolated bacterium was not resistant to the ampicillin and the antibiotic treatment was effective for this strain. Moreover, the probiotic also plays a therapeutic role and can improve urinary infections.
Introduction
Escherichia coli (E.coli) is an anaerobic Gram-negative bacterium from the Enterobacteriaceae family that commonly is present in warm-blooded and cold-blooded animals. Most E.coli strains are harmless [1]. E.coli bacteria is a part of the natural intestinal flora. This type of bacterium occupies 0.1% of the intestinal flora [2] and prevents the establishment of Enterobacteriaceae and some proteolytic bacteria in the intestine. However, some serotypes such as O157: H7 cause food poisoning and diarrhea. E.coli has been known as the most important cause of urinary tract infections (UTIs), accounting for about 90% of UTIs in young women [3].
Urinary Tract Infection (UTI) is a collective term that describes any infection involving any part of the urinary tract, namely the kidneys, ureters, bladder, and urethra. The urinary tract can be divided into the upper (kidneys and ureters) and lower tract (bladder and urethra) [4].
In general, 40% of women develop a UTI at some point in their life. In Singapore, 4% of young adult women are affected and the incidence increases to 7% at 50 years of age [5]. Adult women are 30 times more likely than men to develop a UTI, with almost half of them experiencing at least one episode of UTI during their lifetime [6]. It is reported that one in three women have their first episode of UTI by the age of 24 years [6]. UTIs are most commonly seen in sexually active young women. Other susceptible adults include the elderly and patients requiring urethral catheterization [7].
Antibiotic resistance is known as one of the most serious public health problems throughout the world. According to the reports, the appropriate antibiotic for the treatment of UTIs should be selected based on the pattern of resistance in different geographical areas, the drug availability, and the patients’ history of non-allergy. This study makes an attempt to investigate the bacteria belonging to the Enterobacteriaceae family [8].
Given the resistance of pathogenic bacteria to antibiotic drugs, one of the ways to treat and overcome the antibiotic resistance problem is to make use of other treatment methods and consider natural substances, e.g., probiotic bacteria, for their treatment [9]. In this field, there is not much research conducted on the probiotic activity of bacterial species, especially in UTIs, and the use of live microorganisms (probiotics) can be a promising alternative for the prevention and treatment of frequent UTIs [10].
Generally, the first way to deal with UTIs, especially uropathogenic E.coli-induced infections, is treatment with antibiotics. However, today it has been established that due to the formation of biofilm as well as the emergence of antibiotic resistance in these bacteria, the use of antibiotic treatment has been limited and many studies have focused on finding alternative ways for treatment. One of the alternative ways is anti-adhesion therapy, which is a method to block bacterial adhesion and prevent bacterial biofilm formation.
Among the other methods tested in this field in the last few decades, is the use of commensal or beneficial microorganisms, e.g., probiotic lactobacilli, to compete and prevent the adhesion of pathogenic bacteria [11,12]. Probiotic bacteria, e.g., probiotic lactobacilli, compete with pathogenic microorganisms in binding to epithelial surfaces and have specific and non-specific binding ability for target areas. Specific binding occurs when a bacterial cell surface adhesion binds to a host epithelial cell surface receptor. This action is known as a lock-and-key function. Non-specific binding of probiotic bacteria is a more common phenomenon caused by hydrophobic or electrostatic force. Although non-specific binding may not be sufficient for establishment on epithelial cells in vivo, it is probably effective in absorbing the substrate, consequently increasing the growth [13,14]. In general, the probiotic lactobacilli interact with pathogens of the genitourinary system in very diverse ways, which is due to the four main characteristics of these bacteria, including the ability to bind, compete, and inhibit uropathogens [15]. When using the probiotic supernatant, the antimicrobial effect of Lactobacillus casei is also higher than that of other lactobacilli, and Lactobacillus casei has a greater aggregation with uropathogenic as well [16]. Since pathogenic microorganisms must first bind to surfaces to cause disease and then lead to disease through their pathogenic factors, this role of probiotic bacteria is one of the ways to deal with pathogenic bacteria [14-17]. One of the important points in the treatment of infectious diseases is to determine antibiotic resistance before starting treatment. It should be noted that excessive use of antibiotics should be avoided in order to prevent the emergence of resistant strains while treating effectively [18]. Considering the wide range of bacteria, one of the concerns of biologists and microbiologists is to find useful and harmless types of bacteria as well as to identify their pathogenic types. In this study, the pathogenicity of E.coli bacteria is investigated using the desired genes, i.e., iutA and papG [2], with the aim of achieving the treatment of E.coli-induced urinary infections and E.coli antibiotic-resistant strains [19]. Considering the presence of various non-pathogenic and pathogenic strains of E.coli in animals, especially in humans, a large number of studies have been done on this bacterium by medical and pharmaceutical researchers. Finding useful and harmless species and identifying the pathogenic and harmful species are among the main concerns of scientists in recent years, which have been investigated with different methods of identifying the biology of different strains [19,20]. Extensive research has focused on the identification of the difference between the strains of this bacterium and their type of activity. However, the probiotic activity of different strains of this bacterium in the treatment of infections, especially UTIs, has been less studied [19]. Considering the wide range of this bacterium in this field, the probiotic activity of the pathogen strain isolated from urinary infections through the PCR method and cell culture has been studied from a molecular perspective [3]. According to the above, by achieving this goal, we can hope for the treatment of E.coli-induced urinary infections and its strains that are resistant to antibiotics. One of the concerns in biological and medical sciences is bacterial and fungal resistance, as the increase in resistance caused by excessive use of chemical antibiotics has increased the resistance of some of these bacteria to chemical drugs by more than 90%. iutA and papG genes are located in the outer membrane of Gram-negative bacteria, including E.coli, and cause their accumulation and binding to periplasmic protein receptors [21]. In this study, the frequency analysis of iutA and papG genes is present in the isolates of the UroPathogenic Escherichia coli (UPEC) infection, and the UPEC has the highest frequency in iutA and papG genes. For this purpose, these genes are used to investigate the pathogenicity of the urinary tract [22]. This investigation aimed at the effect of Lactobacillus casei probiotic on iutA and papG genes in the desired strain of E.coli by using different methods.
Materials and methods
The present article is an experimental study with the exemption code number IR.IAU.CTB.REC.1401.01.
Sample collection
For this study, 40 urine samples were collected from the microbiology unit of the NAJA Imam Sajjad Hospital. The E.coli microbial strain used was obtained from the Pasteur Institute with ATCC number 25922 and was used as a positive control for iutA and papG genes [23].
In addition, the Lactobacillus casei strain with ATCC number 39392 was obtained from the Iranian Microorganism Collection Center. Then, the collected bacterial colonies were evaluated in terms of colony phenotypic characteristics (colony color, colony shape, etc.). Gram-negative bacilli with colored colonies in mechanical agar culture medium (Non-fermenting bacteria are colorless in this environment) as well as colonies with metallic luster in the EMB medium were selected as uropathogenic E.coli strains. To ensure the correctness of the selection of the colonies, biochemical tests were carried out for each of the desired colonies separately:
- Cultivation of colonies in TSI medium to test bacteria in terms of fermentation of sugars (glucose, lactose, sucrose), H2S production, and gas production (The TSI test is a microbiological test named for its ability to test a microorganism's ability to ferment sugars and produce hydrogen sulfide)
- Citrate utilization test in Simmons Citrate medium
- Bacterial motility test (Motility) in SIM medium
- Indole test using Kovac's reagent
- Methyl red (MR) test and Voges–Proskauer (VP) test
- Urease test
Finally, 40 isolates of uropathogenic E.coli (their response to each of the mentioned tests was as described in Table 1) were selected as uropathogenic E.coli strains, and the presence of uropathogenic E.coli was confirmed in all the samples with differential tests, and these samples were preserved for further research. In order to preserve the clinical isolates of E.coli, first, appropriate and sufficient amounts of bacterial colonies near the flame were removed by the use of a sterile needle. Then, they were inoculated with TSB in the maintenance medium. These environments can support the growth of strains for a long time. After bacterial inoculation, 15% glycerol was added to the medium and the samples were transferred to a -70°C freezer. Then, they were inoculated in a TSB medium. These media can support the growth of strains for a long time. After the bacterial inoculation, 15% glycerol was added to the medium and the samples were transferred to a -70 °C freezer. The urine samples were cultured linearly on the culture medium EMB (agar) and poured into the differential tubes in order to detect the presence of E.coli by examining the metal polish. Then, the samples (N = 40) were cultured on the plates. After the cultivation, the samples were transferred to the incubator and a time interval of 24h was considered for the formation of colonies. The samples were removed from the incubator after 24h and transferred to a non-sterile refrigerator. Next, the liquid culture medium was made and the colonies formed on the solid culture medium were cultured on the Hinton Broth liquid culture medium. First, 39.3gr of Hinton Broth culture medium was weighed and dissolved in 30cc of distilled water. 5cc of this solution was poured into Falcon 15 and incubated. After 24h, it was transferred to the incubator and incubated there for 24h as well. In the next step, the DNA was extracted.
The primer used in the Polymer Chain Reaction (PCR) in the present study has been examined for amplification of the iutA and papG genes [25]. First, the DNA of the samples was extracted using the boiling method [24], and then, a specific primer was designed to identify the genes. Designing inappropriate primers can lead to non-amplification of the desired fragment in PCR, low production of PCR products, or on the other hand, non-specific production of PCR products. All the primers used in this study were designed using the Gene Runner software. To assign the designed primers, the above sequence was checked in the NCBI BLAST database, and the degree of overlap and cross-reaction of the other genes and organisms was checked. The specifications of the specific primers used in the PCR are listed in Table 2.
Composition of materials and thermal plan used in PCR
The Polymerase Chain Reaction (PCR) was performed with a final volume of 25 µl, containing 12.5 µl master mix, 0.5 µl forward, 0.5 µl reverse, 2 µl of DNA genome sample, and 5.9 µl sterile distilled water.
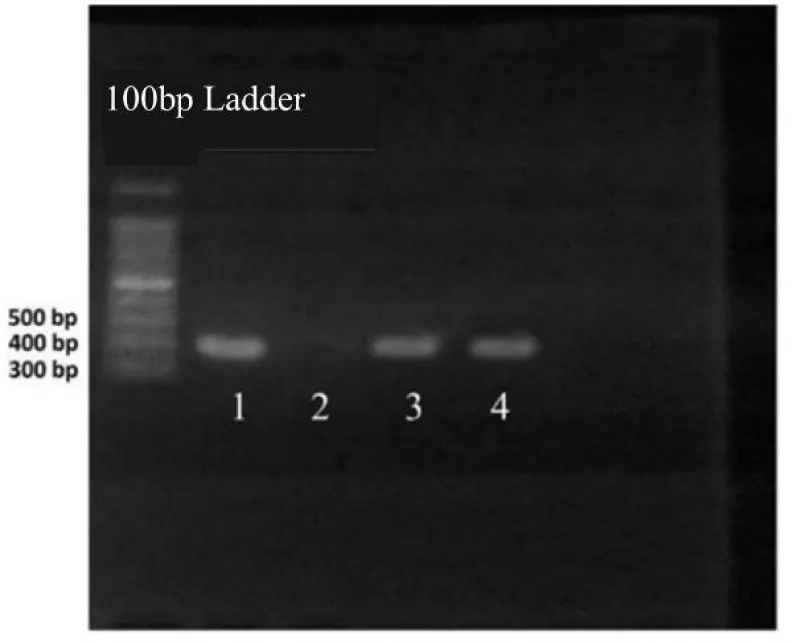
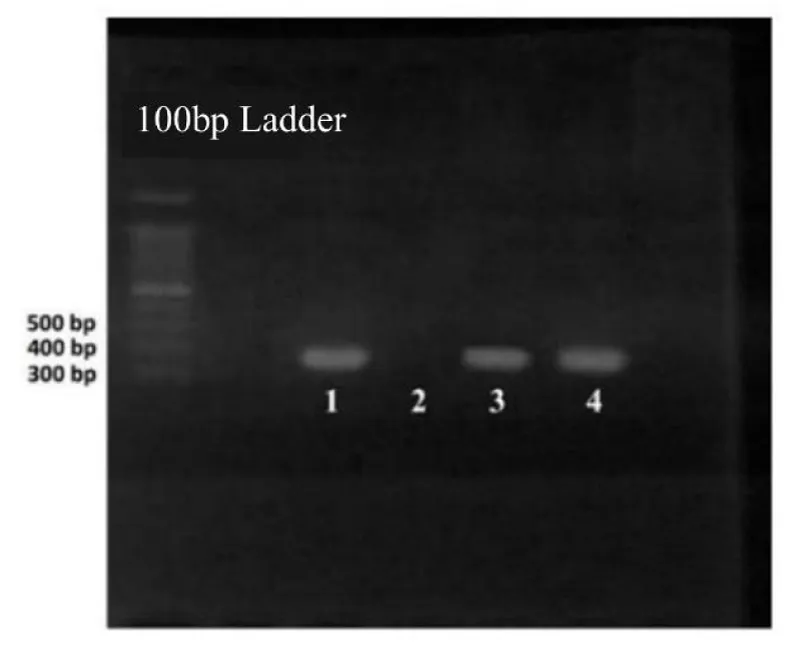
It should be noted that in each series of tests, the PCR of the desired samples, in addition to positive and negative controls (to ensure the absence of contamination in the test), along with unknown PCR samples were given. Then using a Thermal Cycler, a temperature plan including initial denaturation for 10 minutes at 95 °C, denaturation for 45s at 95 °C, splicing the specific primers of iutA and papG genes for 52s at 58 °C for 40 cycles, and final extension for 25s at 72 °C was performed (Table 3). Next, the product obtained from the PCRs was electrophoresed on 1% agarose (well 2, negative control) (well 3, positive control) and was examined using the Gel-Doc device.
Supernatant extraction of Lactobacillus casei isolate was cultured according to its specific growth conditions, and the supernatant was extracted. To ensure the absence of bacteria in the supernatant, a 0.24 µl needle filter was used, through which the supernatant was passed. At first, a fresh culture broth was prepared from the desired bacteria (double positive and double negative) in the Mueller Hinton culture medium. Then, an amount of each bacterium was dissolved in a sterile physiological serum.
In order to derive the bacterial strain of E.coli and confirm the desired strain, the pathogenic E.coli was isolated from the patients with UTI, and gram staining was performed for them. After being confirmed, this strain was used in order to prepare cell suspension for testing the Minimum Inhibitory Concentration (MIC).
The culture supernatant of Lactobacillus cells was used to measure the direct effect of probiotic bacteria. The clinical strain of Lactobacillus casei was obtained from the Iranian Microorganism Collection Center. Mueller Hinton broth medium was used to perform the MIC test. A suspension of bacteria was prepared with a standard amount equivalent to half of McFarland, which is equivalent to 1.5 x 108 cfu/ml of bacteria according to the standard table.
The MIC test for the lethal effect of probiotic bacteria on the desired strain of E. coli was performed by the microplate method in the presence of different dilutions of probiotic bacteria (as an antibiotic) [26] different dilutions including (%, 20%, 25%, 12.5%, 15% 10). It was prepared serially from the antibiotic (probiotic strain) in Mueller Hinton broth in a volume of 1 µl. Different dilutions (i.e., 25%, 20%, 15%, 12.5%, and 10%) were serially prepared from the antibiotic (probiotic strain) in Mueller Hinton broth medium in a volume of 1 µl. At the end, a volume of bacteria present in physiological serum was added to each of the samples, the amount of bacteria in which was equal to 105 µl, and then it was placed inside the incubator for 24h incubation at a temperature of 37 °C. After this time, turbidity caused by bacterial growth was observed in the wells, and the MIC dilutions were read. With the observation of turbidity in each well, the previous well was considered as the MIC.
For the quality control of the samples, a positive control group (culture medium with bacteria) and a negative control group (culture medium and tested material) were considered. After 24h, the turbidity of the bacteria was checked.
The term MBC refers to the minimum concentration that kills bacteria. At this stage, the microbial sensitivity of each of the clinical isolates of E.coli to the antibiotic ampicillin was evaluated. After preparing the supernatant of the 48h culture of Lactobacillus casei bacteria, 40 µl of the supernatant with dilutions of 1, 1.2, 1.4, and 1.8 was added to the specific blank antibiogram discs.
In the disk-diffusion method, first, an initial suspension of probiotic bacteria was prepared in the Mueller Hinton broth medium with a concentration of 4 µg, equivalent to 0.1 McFarland. Then, it was mass-cultured with a sterile swap.
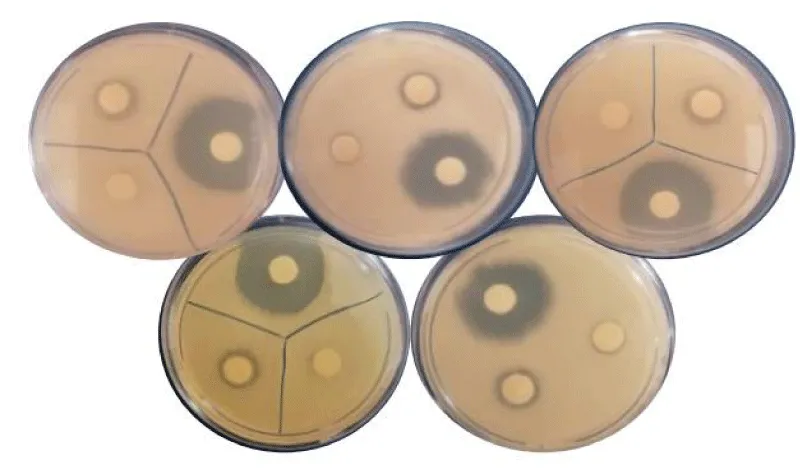
In this study, ampicillin antibiotic disc was used as a positive control. The reason for choosing this antibiotic disc is its broad spectrum and availability, as well as its common use. The discs coated with the tested sample and ampicillin were placed in the agar medium at appropriate distances and incubated for 24h in an incubator at 37 °C. The diameter of the zone of inhibition was measured by a ruler.
Statistical tests were used to analyze the data, the presence or absence of the desired genes in E.coli samples isolated from the UTI patients referred to the laboratory of the microbiology unit of the NAJA Imam Sajjad hospital, and the effect of probiotics on the isolated bacteria. The t-test of the GraphPad-Prism 6,6 software was used for statistical evaluations and all the data were compared together. In the clinical and standard strains of E.coli, the diameter of the zone of inhibition was observed in the presence of the antibiotic (probiotic bacteria) compared to its absence. On the other hand, no significant difference was observed among all the clinical strains compared to the standard strain. This difference was observed only among the clinical strains compared to the standard group, which can be associated with the differences between the clinical strains in some characteristics.
Results
From March 2020-2021, 40 isolates were investigated, from which 40 E.coli isolates were isolated using biochemical tests. Of the 40 samples taken from the patients, 9 were men and 31 were women, of which 28 had symptoms and 12 had no symptoms, 15 of them were under 40 years old and 25 were over 40 years old. It has been their age. This number of continuous sampling has been done over several months from the laboratory of Imam Sajjad (AS) Naja Hospital, Department of Microbiology, of patients with urinary tract infections (Table 4).
Bacteria detection
In this research, the PCR test was used to detect the presence of the desired genes. In addition, the cell culture method was used to confirm the results. The sequence and specifications of the desired primers were obtained using scientific literature, which was used in the PCR. With the help of the PCR device and with the appropriate temperature plan, the target sequence was amplified and then identified by the use of the Agarose gel electrophoresis method. NCBI gene bank information was also used if needed.
The results of molecular tests for the presence of iutA and papG genes in E.coli strains were checked by the use of the PCR technique (Figures 1,2). The standard strain of E.coli (positive control), ATCC25922, was considered as a positive control. According to the PCR results, out of 40 E.coli strains isolated, 8 strains indicated a clear band after the PCR of the papG and iutA genes in agarose gel.
Probiotic bacteria culture test results
In the complete culture test of probiotic bacteria, the average diameter of the zone of inhibition for uropathogenic E.coli by Lactobacillus casei was 9 mm; however, it was reported as 19 mm in the control.
Moreover, the antimicrobial effect of Lactobacillus casei on uropathogenic E.coli in the state of complete culture of probiotic bacteria was much lower than in the same state in antibiograms. In the test to evaluate the antimicrobial effect of the supernatant, the turbidity of the tubes in the uropathogenic E.coli samples indicated that the average MIC of the Lactobacillus supernatant is less than 9. However, this value was greater than 19 in the control group. The results of this study indicated that in the disk-diffusion method, the antimicrobial effect of probiotics was significantly dependent on the dilution. In higher dilutions of probiotic bacteria, the diameter of the zone of inhibition was smaller (Table 5).
Results of MBC-MIC tests
The MIC-MBC test included different dilutions of the supernatant, i.e., 25%, 20%, 15%, 12.5%, and 10%, prepared from probiotic bacteria. In this test, these dilutions were used directly, and the Lactobacillus casei strain prepared with a concentration equivalent to half McFarland was examined after about 24h of incubation (Table 6).
Antibiotic sensitivity test results
Diffusion-disk test report: As can be seen in Figure 3, the inhibitory effect of antibiotic disc with a concentration of 4 µg was investigated on 5 samples isolated from the UTI patients. The zone of inhibition was investigated separately and its diameter was reported to be in the range of 0 mm - 10 mm. Then, the diameter of the zone of inhibition was measured by a ruler, as listed in Table 5.
Discussion
According to the results from this study about the prevalence of the genes considered, it can be concluded that the prevalence of these genes is high among UTI patients. The PCR method is an efficient tool for rapid diagnosis and detection of pathogenic agents of urinary infections in E.coli. At the same time, in this study, the most effective antibiotic against the studied isolates is imipenem; however, in case of excessive prescription, resistance to this antibiotic is likely to occur as well. UTI is known as the second most prevalent infection after respiratory infection. Due to the ease of getting a urinary infection and its very dangerous complications, e.g., kidney failure, blood infection, premature birth, timely diagnosis and treatment of this infection is of high importance. Just as for many infectious diseases, the treatment of UTI requires the doctor to identify the cause of the infection and determine the sensitivity of the bacterial agent to antibiotic agents before starting the treatment. The aim of this study was to investigate the expression of iutA and papG genes in E.coli O157:H7 strains isolated from urinary infection samples from the patients referred to the laboratory. Due to the limitation in the number of samples and the measurement of the samples, our results cannot be used for a wide-range statistical conclusion. Besides, considering the complications and mortality caused by pathogenic bacteria, yeasts, and viruses, people are often concerned about general microbes. However, currently, a collection of evidence shows that probiotic bacteria can help human health [27,28]. According to research, food supplements, e.g., some probiotics, have emerged as a valid option to control many diseases [29]. Therefore, many researchers are trying hard to find safe and natural antibiotics such as probiotics [30]. At present, the use of probiotics is considered an alternative to antibiotic-resistant and food-spoilage microorganisms [31]. Of course, it has been stated in some cases, that only if the culture media contains concentrated probiotic bacteria or is at a certain pH, its inhibitory effect can be observed [32]. Factors such as pH level have a great effect on the binding of probiotics in destroying the zone of inhibition [33]. Therefore, the present study made an attempt to investigate the inhibitory effect of probiotic bacteria on E.coli bacteria and clinical strains. The important feature of probiotic bacteria is their ability to inhibit the increase in the number of pathogenic microorganisms as well as to inhibit their pathogenicity. Lactic acid bacteria, e.g., Lactobacillus genus, play a very effective role in this regard by producing antimicrobial agents and using different mechanisms [34].
Lactiplantibacillus plantarum and Lactobacillus casei are other bacteria affecting E.coli-induced urinary infection. These two bacteria can trap and accumulate with the uropathogenic E.coli bacteria, causing a decrease in binding and preventing its biofilm formation in laboratory conditions [35].
The main bacteria obtained include E.coli, Klebsiella, Enterobacter, Citrobacter, Pseudomonas, and Staphylococci. Most of these bacteria are the natural flora of the body, and common antibiotics such as cotrimoxazole and ciprofloxacin are known to play a significant role in the treatment of E.coli bacteria [36]. In the last decade, many studies have been conducted in different regions of the world on the isolation and investigation of the frequency of UTI agents using different techniques, including the culture method and the molecular diagnostic method.
Grozdanov, et al. [37] studied the genome structure of the non-pathogenic probiotic E.coli Nissle 1917. In their research, they investigated different parts of the chromosomes of pathogenic and non-pathogenic strains of E.coli through the tRNA analysis method and Evaluated Genomic Islands (GEIs) and DNA by the use of the DNA/DNA hybridization method. Their evidence indicated that different iron absorption systems, adhesives, and proteases can help the successful survival of this probiotic strain in the human intestine, which is most likely to cause the destruction of E.coli [37].
Falagas, et al. [20] investigated the role of probiotic bacteria in preventing UTIs in women. Frequent UTIs have affected a large number of women throughout the world. The use of probiotics, especially lactobacilli, is considered to prevent UTIs. Besides, UTI is more frequent in postmenopausal women and causes morbidity in them. Researchers have suggested restoring the urinary flora dominated by pathogens using lactobacilli in order to protect against UTIs. Many studies involving animal and microbiological tests have been conducted to evaluate and investigate the effectiveness and safety of probiotics against uropathogens in healthy women with UTIs. Many of these studies achieved inspiring findings for some specific strains of lactobacilli. According to the results, it seems that among the studied lactobacilli, Lactobacillus rhamnosus, GR-1, and L. reuteri RC- 14 (previously called L. fermentum RC-14) are more effective in preventing UTIs.
L. crispatus CTV-05 and L. Casei shirota have also proved their effectiveness in some studies. L rhamnosus GG does not seem to be as effective as them in preventing UTIs. Evidence from the available studies has shown that probiotics can play an effective role in preventing frequent UTIs in women. Moreover, they have good safety features. However, more research is needed in this regard [20].
In their study, Fijan, et al. [38] investigated the molecular activity of different single-chain and multi-chain E.coli probiotic strains. The strong resistance of pathogenic strains of E.coli to many antibiotics has made studies on this bacterium of great interest to researchers. One of the ways to deal with pathogenic and resistant strains of E.coli is to use probiotic strains. The aim of this research is to investigate and evaluate the activity of antagonist agents using single-chain and multi-chain probiotic products against E.coli clinical pathogens [38].
Ahmadi, et al. (2017) in a study investigated the prevalence of virulence genes in E.coli bacteria isolated from general cases of poultry bacillus and human UTI. The prevalence of 4 virulence-related genes, i.e., tsh, traT, iutA, sitA, in 26 APEC isolates and 25 UPEC isolates isolated from clinical cases suspected of E.coli infection in West Azerbaijan was investigated by the use of the PCR technique. The statistical analysis was done using the Minitab 15.0 software. In the case of pathogenic isolates in human UTI, the iutA-sitA-traT. combination has the highest frequency, there is a possibility that virulence genes are transferred between poultry and humans. It is possible that virulence genes are transferred between poultry and humans [22].
Nemati, et al. (2013) investigated the frequency of uropathogenic Ecoli causing UTIs and determined some virulence genes in isolates isolated from the patients referred to the Shahid Beheshti Hospital in Kashan during the period 2012-2013. They focused on the study of the virulence genes hly, pap, aer, pai, and traT. In the following, a collection of 150 Escherichia coli isolates was isolated from 370 urine samples collected from patients admitted to Shahid Beheshti Hospital in Kashan. Nest, a collection of 150 E.coli isolates was isolated from 370 urine samples from the patients admitted to the Shahid Beheshti Hospital in Kashan. The E.coli bacteria were identified using standard biochemical and microbiological techniques, and the prevalence of virulence factors was investigated using the PCR method. The results indicated that there is a high prevalence of traT, pai, and aer virulence genes among the uropathogenic E.coli strains isolated from the hospitalized patients in the study. Therefore, the above genes can be further investigated as a target in therapeutic interventions [39].
Rashki, et al. investigated the relationship between phylogenetic groups and the distribution of virulence genes in E.coli isolates isolated from UTI patients in order to determine the frequency of genes encoding virulence factors and their relationship with the phylogenetic group with the mentioned patients [40]. This descriptive-analytical study was conducted on 100 E.coli isolates isolated from the UTI patients. The presence of the genes encoding virulence factors was checked by the use of the Multiplex-PCR method. In addition, the phylogenetic groups including A, B1, B2, and D were determined based on the presence or absence of the triple-PCR genes using the TspE4.C2 methods and yjaA and chuA fragments. Among the isolates of group B2, virulence genes are more widespread than the other phylogenetic groups [40].
Alishah, et al. [41] investigated the antibiotic resistance and detection of papG and PapC genes in E.coli bacteria isolated from the UTI. In their cross-sectional descriptive study, 50 UTI children referring to Tehran Medical Center were selected, and after bacteria isolation and DNA extraction, the necessary tests were performed and the presence of papG and PapC class genes was checked using the Multiplex-PCR method. The results indicated that PapC and papG genes of class I are the most prevalent genes encoding pap fimbriae adhesin in E.coli isolated from the UTI patients. The reason for the difference between the results of their research and other studies is due to the diversity of the geographical region [41].
Shirazi, et al. [42] investigated the cloning of the papG gene of uropathogenic E.coli and its sequence diversity. Uropathogenic E.coli is the predominant pathogen in UTIs. Despite the various antigens and toxins of bacteria involved in causing infection, one of the important factors in E.coli-induced infections and other Gram-negative bacteria is the adhesion of bacteria to the surface of the host cell, so inhibiting bacterial adhesion is a suitable solution to control this type of infection. Considering that the papG protein acts as an adhesion factor, the N-terminal area of papG between clinical strains is a conserved sequence that can be used to design a vaccine against UTIs [42].
Beyitler, et al. (2017) In the research entitled Probiotics for Prophylaxis and Treatment of Urinary Tract Infections in Children. Probiotics inhibit uropathogens by competition for receptors and nutrients, direct killing, immune modulation, and production of inhibitory metabolites. There are many organisms that have been used as probiotics. Lactobacillus sp., Bifidobacterium sp., and Saccharomyces boulardii are the most commonly used and investigated probiotics. Although there are various benefits of probiotics for the pediatric population, some reports indicate rare complications such as bacteremia, sepsis, endocarditis, meningitis, UTI, abscesses, fungemia, pneumonia, and chorioamnionitis. However, these are much less than the benefits of probiotics yet should be kept in mind. Continuing laboratory and clinical studies are encouraging the use of this strategy for the prevention and treatment of UTIs in children [43].
In a study conducted by Akgül in 2018. In studies on urinary microbiomes, the degree of concentration of bacteria habiting in urinary systems may vary according to sex, the method of urine collection, and the technique used to study UM. In general, Lactobacillus and Streptococcus are the most frequently observed species and many studies have been performed on them. Both microorganisms are lactic acid bacteria and have protective roles against pathogens colonized in the urogenital region [44]. Other less frequently found bacterial strains are Alloscardovia, Burkholderia, Jonquetella, Klebsiella, Saccharofermentans, Rhodanobacter, and Veillonella [45].
The beneficial effects of using probiotics due to the development of beneficial intestinal bacteria or the decreased pathogenicity of harmful microbes are very significant in ensuring the body's health and especially the digestive system's health. Given the direct effect of the digestive system on the immune system, probiotics can strengthen the immune system by producing more lymphocytes and preventing the occurrence of infectious diseases. The use of probiotics can reduce and prevent the absorption of allergenic substances in dairy products through the intestine. Probiotics are able to inhibit the development of pathogenic bacteria, including E.coli. In recent years, resistance to prevalent and effective antibiotics in the treatment of infectious diseases has become one of the most important challenges [46]. Antibiotic resistance is important because in many cases when the disease occurs and antibiotic treatment is required, the resistant microorganisms do not respond to the treatment [47]. In this study, we have shown that probiotics can have a positive effect on infections, especially urinary infections, and probiotics can be a suitable alternative to antibiotics in the near future, but to prove it, we need Studies with a larger sample size.
Conclusion
One of the most important aspects of this study is the lack of resistance to the antibiotic used as well as the observation of the zone of inhibition through this antibiotic. The probiotic used in this study also had synergistic effects for this antibiotic, which indicates that these probiotics can replace antibiotics by inducing some mechanisms.
- pour GZ, Salehzadeh A, Zamani H. Prevalence of Pathogenicity Islands and Fim H Virulence Genes in Escherichia coli Strains Isolated from Urinary Tract Infection in Rasht City, Iran. Journal title 2018; 26 (3):63-71
- Mehryari A, Parviz M, Khalajzadeh S. Identifying and determining the Classes I, II, and III papG Gene of Escherichia Coli Isolated from Patients with Urinary Tract Infections. Journal of Isfahan Medical School. 2015; 33(348): 1412-1419.
- Mehdizadeh M, Eskandari SO, Zavar M, Piroz B. The Importance of Escherichia coli O157: H7 in Foodborn Infection. Journal of Kerman University of Medical Sciences. 2008 Sep 1;15(4):353-61.
- Tan CW, Chlebicki MP. Urinary tract infections in adults. Singapore Med J. 2016 Sep;57(9):485-90. doi: 10.11622/smedj.2016153. PMID: 27662890; PMCID: PMC5027397.
- Tan CW, Chlebicki MP. Urinary tract infections in adults. Singapore Med J. 2016 Sep;57(9):485-90. doi: 10.11622/smedj.2016153. PMID: 27662890; PMCID: PMC5027397.
- Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002 Jul 8;113 Suppl 1A:5S-13S. doi: 10.1016/s0002-9343(02)01054-9. PMID: 12113866.
- Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011 Oct;5(5):316-22. doi: 10.5489/cuaj.11214. PMID: 22031610; PMCID: PMC3202002.
- Khawcharoenporn T, Vasoo S, Singh K. Urinary Tract Infections due to Multidrug-Resistant Enterobacteriaceae: Prevalence and Risk Factors in a Chicago Emergency Department. Emerg Med Int. 2013;2013:258517. doi: 10.1155/2013/258517. Epub 2013 Oct 31. PMID: 24307946; PMCID: PMC3844142.
- Talebi TM, Niksolat M, Minaeian S, Khodabandelou N, Zandieh Z. The effect of probiotics in the prevention of urinary tract infections in elderly patients hospitalized in intensive care units. 2017; 24(156):32-41.
- Borchert D, Sheridan L, Papatsoris A, Faruquz Z, Barua JM, Junaid I, Pati Y, Chinegwundoh F, Buchholz N. Prevention and treatment of urinary tract infection with probiotics: Review and research perspective. Indian J Urol. 2008 Apr;24(2):139-44. doi: 10.4103/0970-1591.40604. PMID: 19468386; PMCID: PMC2684288.
- Da Silva GJ, Mendonça N. Association between antimicrobial resistance and virulence in Escherichia coli. Virulence. 2012 Jan-Feb;3(1):18-28. doi: 10.4161/viru.3.1.18382. Epub 2012 Jan 1. PMID: 22286707.
- Ponnusamy P, Natarajan V, Sevanan M. In vitro biofilm formation by uropathogenic Escherichia coli and their antimicrobial susceptibility pattern. Asian Pac J Trop Med. 2012 Mar;5(3):210-3. doi: 10.1016/S1995-7645(12)60026-1. PMID: 22305786.
- Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, Gómez-Llorente C, Gil A. Probiotic mechanisms of action. Ann Nutr Metab. 2012;61(2):160-74. doi: 10.1159/000342079. Epub 2012 Oct 2. PMID: 23037511.
- Hütt P, Shchepetova J, Lõivukene K, Kullisaar T, Mikelsaar M. Antagonistic activity of probiotic lactobacilli and bifidobacteria against entero- and uropathogens. J Appl Microbiol. 2006 Jun;100(6):1324-32. doi: 10.1111/j.1365-2672.2006.02857.x. PMID: 16696680.
- Asahara T, Nomoto K, Watanuki M, Yokokura T. Antimicrobial activity of intraurethrally administered probiotic Lactobacillus casei in a murine model of Escherichia coli urinary tract infection. Antimicrob Agents Chemother. 2001 Jun;45(6):1751-60. doi: 10.1128/AAC.45.6.1751-1760.2001. PMID: 11353622; PMCID: PMC90542.
- Sadri M, Soleimani AN, Forghanifard MM. The study of Antimicrobial and Anti-adhesive effect of ProbioticLactobacilli on Uropathogenic Escherichia coli (UPEC). Biological Journal of Microorganism. 2016 May 21;5(17):159-70.
- Watson RR, Preedy VR, editors. Probiotics, prebiotics, and synbiotics: bioactive foods in health promotion. Academic Press. 2015 Sep 23.
- Shawar RM, MacLeod DL, Garber RL, Burns JL, Stapp JR, Clausen CR, Tanaka SK. Activities of tobramycin and six other antibiotics against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob Agents Chemother. 1999 Dec;43(12):2877-80. doi: 10.1128/AAC.43.12.2877. PMID: 10582875; PMCID: PMC89580.
- Keikha M, Rava M. Trend of antibiotic resistance of Escherichia coli strains isolated from urinary tract infections in outpatient patients from Zahedan. Journal of Paramedical Sciences & Rehabilitation. 2017 Dec 22;6(4):73-8.
- Falagas ME, Betsi GI, Tokas T, Athanasiou S. Probiotics for prevention of recurrent urinary tract infections in women: a review of the evidence from microbiological and clinical studies. Drugs. 2006;66(9):1253-61. doi: 10.2165/00003495-200666090-00007. PMID: 16827601.
- Nolan LK, Barnes HJ, Vaillancourt JP, Abdul-Aziz T, Logue CM. Colibacillosis in: Swayne D.E. (Eds) Diseases of poultry. Wiley-Blackwell, 13th edition. 2013; 751-805.
- Ahmadi M, Dadashzadeh S, Ghaniei A. Prevalence of virulence genes in Escherichia coli isolates implicated in poultry colibacillosis and human urinary tract infection, Journal of Veterinary Microbiology. 2019; 15(1): 109-118.
- Schouler C, Schaeffer B, Brée A, Mora A, Dahbi G, Biet F, Oswald E, Mainil J, Blanco J, Moulin-Schouleur M. Diagnostic strategy for identifying avian pathogenic Escherichia coli based on four patterns of virulence genes. J Clin Microbiol. 2012 May;50(5):1673-8. doi: 10.1128/JCM.05057-11. Epub 2012 Feb 29. PMID: 22378905; PMCID: PMC3347144.
- Johnson JR, Brown JJ. A novel multiply primed polymerase chain reaction assay for identification of variant papG genes encoding the Gal(alpha 1-4)Gal-binding papG adhesins of Escherichia coli. J Infect Dis. 1996 Apr;173(4):920-6. doi: 10.1093/infdis/173.4.920. PMID: 8603972.
- de Lorenzo V, Bindereif A, Paw BH, Neilands JB. Aerobactin biosynthesis and transport genes of plasmid ColV-K30 in Escherichia coli K-12. J Bacteriol. 1986 Feb;165(2):570-8. doi: 10.1128/jb.165.2.570-578.1986. PMID: 2935523; PMCID: PMC214457.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing Twenty-Third Informational Supplement. M100- S23.Pennsylvania: Clinical and Laboratory Standards Institute. 2013.
- Sarikhani Z, Nazari R, Nateghi Rostami M. First report of OXA-143-lactamase producing Acinetobacter baumannii in Qom, Iran. Iran J Basic Med Sci. 2017 Nov;20(11):1282-1286. doi: 10.22038/IJBMS.2017.9490. PMID: 29299207; PMCID: PMC5749364.
- Dudek-Wicher R, Junka A, Paleczny J, Bartoszewicz M. Clinical Trials of Probiotic Strains in Selected Disease Entities. Int J Microbiol. 2020 May 28;2020:8854119. doi: 10.1155/2020/8854119. Erratum in: Int J Microbiol. 2021 Feb 11;2021:7356890. PMID: 32565816; PMCID: PMC7292209.
- Liu Y, Tran DQ, Rhoads JM. Probiotics in Disease Prevention and Treatment. J Clin Pharmacol. 2018 Oct;58 Suppl 10(Suppl 10):S164-S179. doi: 10.1002/jcph.1121. PMID: 30248200; PMCID: PMC6656559.
- Joint FA. WHO working group report on drafting guidelines for the evaluation of probiotics in food. London, Ontario, Canada. 2002 Apr 30;30.
- Chatterjee M, Anju CP, Biswas L, Anil Kumar V, Gopi Mohan C, Biswas R. Antibiotic resistance in Pseudomonas aeruginosa and alternative therapeutic options. Int J Med Microbiol. 2016 Jan;306(1):48-58. doi: 10.1016/j.ijmm.2015.11.004. Epub 2015 Nov 28. PMID: 26687205.
- López-Brea M, Alarcón T, Domingo D, Díaz-Regañón J. Inhibitory effect of Gram-negative and Gram-positive microorganisms against Helicobacter pylori clinical isolates. J Antimicrob Chemother. 2008 Jan;61(1):139-42. doi: 10.1093/jac/dkm404. Epub 2007 Oct 26. PMID: 17965421.
- Yang E, Fan L, Jiang Y, Doucette C, Fillmore S. Antimicrobial activity of bacteriocin-producing lactic acid bacteria isolated from cheeses and yogurts. AMB Express. 2012 Sep 10;2(1):48. doi: 10.1186/2191-0855-2-48. PMID: 22963659; PMCID: PMC3488010.
- Sadri M, Soleimani AN, Forghanifard M. The study of Antimicrobial and Anti-adhesive effect of ProbioticLactobacilli on Uropathogenic Escherichia coli (UPEC). Biological Journal of Microorganism. 2016; 5(17): 159-170.
- Bandari S, Soleimani NA, Tajbakhsh E. The effect of probiotic lactobacilli on the attachment power and biofilm formation of Escherichia coli isolated from urinary tract infections. J of Microbial Word. 2018;11(3): 278-287.
- Nourouzi J, Kargar M, Pourshahian F, Kamali M. Study on the prevalence of urinary tract infection by Escherichia coli, antibiotic resistance and plasmid profile of isolated bacteria in Jahrom city. 2006:745-749.
- Grozdanov L, Raasch C, Schulze J, Sonnenborn U, Gottschalk G, Hacker J, Dobrindt U. Analysis of the genome structure of the nonpathogenic probiotic Escherichia coli strain Nissle 1917. J Bacteriol. 2004 Aug;186(16):5432-41. doi: 10.1128/JB.186.16.5432-5441.2004. PMID: 15292145; PMCID: PMC490877.
- Fijan S, Šulc D, Steyer A. Study of the In Vitro Antagonistic Activity of Various Single-Strain and Multi-Strain Probiotics against Escherichia coli. Int J Environ Res Public Health. 2018 Jul 20;15(7):1539. doi: 10.3390/ijerph15071539. PMID: 30036977; PMCID: PMC6069398.
- Neamati F, Firoozeh F, Saffary M, Mousavi SG. The prevalence of uropathogenic E. coli and detection of some virulence genes isolated from patients referred to Kashan Shahid-Beheshti Hospital during 2012-2013. KAUMS Journal (FEYZ). 2014 Jun 10;18(3):267-74.
- Abdi HA, Rashki A. Relationship between phylogenetic group and distribution of virulence genes of Escherichia coli isolated from patients with urinary tract infection. Journal of Gorgan University of Medical Sciences. 2015 Jun 10;17(2):92-7 .
- Alishah M, Zahraei Salehi T, Amini K, Yahya Raeyat R. Evaluation of Antibiotic Resistance and Detection of PapC and papG genes in Escherichia coli Strains Isolated from Patients with Urinary Tract Infection. Qom University of Medical Sciences Journal. 2016 Nov 10;10(8):80-7.
- Hamidiyeh F, Shirazi MH, Fallah Mehrabadi J, Pourmand M, Ostad Mohammadi S, Molla agha Mirzaei H, Afshar D. papG Gene cloning, Escherichia coli uropathogen and examination of its subsequence diversity. ISMJ. 2013 Apr 4;16(1):1-8.
- Salih K. Probiotics for Prophylaxis and Treatment of Urinary Tract Infections in Children. 2017.
- Meghan PM. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. MBio. 2014; 5:4; 10:1128/mbio; 01283-14.
- Akgül T, Karakan T. The role of probiotics in women with recurrent urinary tract infections. Turk J Urol. 2018 Sep;44(5):377-383. doi: 10.5152/tud.2018.48742. Epub 2018 Sep 1. PMID: 30487041; PMCID: PMC6134985.
- Karami P, Aslani MM, Najafi Mosleh M, Alikhani MY. Determination Pattern of Antibiotic Resistance in Entropathogenic Escherichia coli Strains Isolated from Children with Diarrhea. Avicenna Journal of Clinical Medicine. 2012 Jun 15;19(1):27-31.
- Boniadian M, Habibian R, Barati S, Jostejo T. Investigation the antibiotic resistance of the Escherichia coli isolated from gastroenteritis cases in Shahrekord county. Journal of Shahrekord University of Medical Sciences. 2014 Feb 15;15(6):117-23.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
