Open Journal of Asthma
Is asthma over-diagnosed in Cyprus? A clinical study at the outpatient’s primary care level
Charis Armeftis1, Marinos Lemessios1, Christos Anastasiades2, Christina Gratziou3, Nikolaos Siafakas4*, Paraskevi Katsaounou5 and Petros Bakakos6
2Department of Statistics MSc, Greece
3Professor of Pulmonology, National and Kapodistrian University of Athens, Greece
4Emeritus Professor of Thoracic Medicine, University of Crete, Greece
5Associate Professor of Pulmonology, National and Kapodistrian University of Athens, Greece
6Professor of Pulmonology, National and Kapodistrian University of Athens, Greece
Cite this as
Armeftis C, Lemessios M, Anastasiades C, Gratziou C, Siafakas N, et al. (2022) Is asthma over-diagnosed in Cyprus? A clinical study at the outpatient’s primary care level. Open J Asthma 6(1): 001-007. DOI: 10.17352/oja.000017Copyright License
© 2022 Armeftis C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: Although asthma is a common disease accurate diagnosis is missing and it has been reported that often it is over or under-diagnosed.
Aim: To investigate if a physician’s diagnosis of asthma in Cyprus is correct by using a structured algorithm at the outpatient primary care level.
Subjects and Methods: Sixty adults with a self-reported physician diagnosis of asthma, mean age of 47,8 years (29 males and 31 females) were included in the study. Medical history and physical examination, pre-post bronchodilation spirometry and methacholine bronchial challenge test was used to confirm or rule out the diagnosis as well as a three months follow-up. In addition, the cost of treatment was estimated.
Results: Sixteen subjects (27%) had a positive pre-post bronchodilation spirometric test and were considered asthmatics. In 9 out of the 44 remaining subjects a positive Methacholine provocation test confirmed the diagnosis of asthma. The rest of the subjects (n = 35) went into a 3 months observational period during which only 2 showed asthmatic symptoms and were considered asthmatics by a second methacholine test that confirmed the diagnosis. Therefore, a correct asthma diagnosis was established in only 27(45%) of subjects. The annual average cost of medication for asthma confirmed the group was 313 euro/patient (171-454, 95% CI) and the average 2-year unnecessary (asthma ruled-out group) cost of treatment was approximately 297 euro/patient. (179-415, 95% CI).
Conclusions: Physician-diagnosed asthma overestimates the actual prevalence of disease in adults in Cyprus since it was shown that more than half of the participants did not have Asthma. These individuals consume unneeded medications at a significant cost. Thus, the correct diagnosis of Asthma should be made by using more specific tests starting at the primary care level.
Introduction
Asthma is characterized by chronic airway inflammation that results in respiratory symptoms of wheeze, dyspnea, chest tightness, or a cough that varies over time and in intensity, together with variable expiratory airflow limitation [1]. It is estimated to affect 30-50 million people in Europe, approximately 5-10% of all Europeans [2]. The prevalence of asthma varies widely around the world, ranging from 0.2% to 21.0% in adults and from 2.8% to 37.6% in 6- to 7-year-old children [3].
The increase in asthma symptoms and prevalence in different continents and areas indicate that the global burden of asthma continues to rise, but the global prevalence differences are lessening [4-6].
Asthma is not only associated with patient-specific impairment, but it also imposes a significant economic burden on the family and society. Productivity loss is another underappreciated source of economic loss [7]. The direct cost of asthma care in Europe is estimated at 17.7 billion Euros per year [7-9].
The diagnosis of asthma in the community can be difficult since asthma presents with respiratory symptoms that are common to a wide range of other diseases thus, more objective tests can be helpful. Making the distinction between asthma and “pseudo asthma” is an important issue for any health system. Instructions for how to establish the correct diagnosis of asthma had been reported in the GINA 2022 document suggesting an efficient series of tests to diagnose new asthma, starting with pre- and post-bronchodilator spirometry, and if spirometry is inconclusive, further tests should be ordered. Additional tests include bronchial challenge tests, exercise testing, monitoring of peak flow rates, assessments of inter-visit variability in FEV1, or a significant increase in FEV1 after 4 weeks of anti-inflammatory treatment.
If the objective testing does not support a diagnosis of asthma, repeating the tests at a later date or considering alternative tests is suggested. The current guidelines suggest using bronchial provocation testing where asthma is suspected, and prior investigations have been non-diagnostic [10]. Methacholine airway responsiveness is one of the most sensitive and specific asthma diagnostic tests [11]. In the setting of ongoing clinical symptoms, a negative result to a test with relatively high sensitivity, such as an MCT (methacholine challenge test), may be most helpful in making current asthma unlikely [12].
Despite the above simple algorithms that help the physician in outpatient clinical practice to establish accurately the diagnosis of asthma, there is a significant number of studies demonstrating that asthma is often misdiagnosed (over-under) even in advanced Health Systems [13].
In Cyprus, the diagnosis of Asthma is usually made by various Specialists such as General Practitioners, Internists and Pulmonologists. Thus, there is no information concerning the accuracy of the Physician’s diagnosis of asthma in Cyprus especially in the outpatient clinical practice therefore, we designed the present study with the primary objective to determine whether the diagnosis of current asthma in the primary clinical practice could be confirmed or ruled out.
Methods
Study population
The study population consisted of 60 adult subjects with asthma-like symptoms recruited from a population that visit a pulmonary physician’s office at an outpatient clinical practice in Cyprus from 2017 to 2018, (before the Covid-19 pandemic). All subjects had a history of “asthma” based on a previous physician’s diagnosis and were treated with asthma medications.
Patients were excluded from the study if they were smokers, had a respiratory infection in the last four weeks, were using long-term oral steroids, were pregnant, breastfeeding, unable to perform spirometry; or if a bronchial challenge test was contraindicated [14].
Other demographics of the subjects of the study population are presented in Table 1 (Total Group).
Research protocol
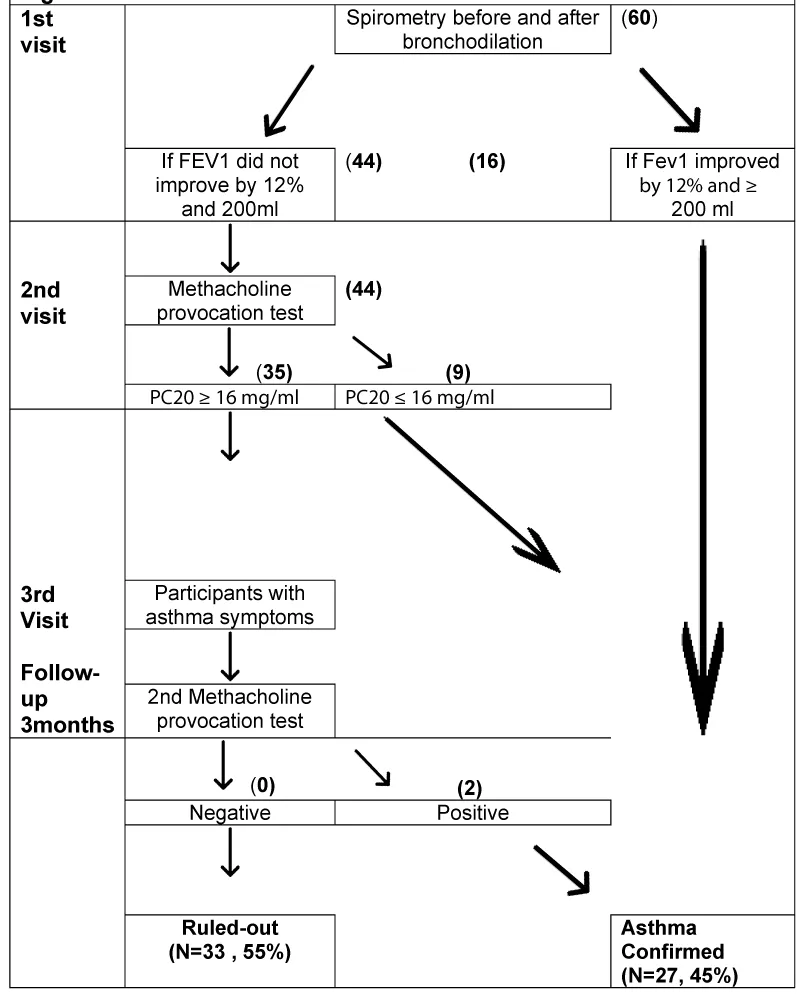
Figure 1 is a flowchart demonstrating the protocol followed in the study: A detailed medical history with current and previously referred respiratory symptoms was taken at visit 1. All the participants were asked to define the physician’s specialty who made the primary diagnosis, for how long they had this diagnosis, what asthma medications or other medications they were using at what dosages, and for how long. In addition, a physical examination was performed. Thereafter, they performed pre-bronchodilator spirometry according to American Thoracic Society standards, instructed by the same Pulmonologist using the same device Medisoft ExpAir 1,29 (01 #29). Post-bronchodilator spirometry was assessed 15 minutes later after administration of 400 μg salbutamol given by a pressurized metered dose inhaler with a spacer device. Patients whose forced expiratory volume in the first second of expiration (FEV1) improved by at least 12% and at least 200 mL after bronchodilation was considered to have reversible airflow obstruction characteristic of current asthma and the diagnosis was established without further tests.
Patients who did not exhibit reversible airflow obstruction had a 2nd visit, where they had a specific bronchial provocation test with provocholine (methacholine chloride, Methapharm Inc). They were asked to withhold inhaled steroids for 15 days, short-acting β-agonists in conventional inhaled doses of at least 6h, long-acting β-agonists (e.g., salmeterol) for 36 h, ultra-long-acting β-agonists (e.g., indacaterol, vilanterol, olodaterol) 48h, Ipratropium (Atrovent 40 μg) 12h, long-acting anti-muscarinic agents for >16818h, Oral theophylline12–24 h [12].
Contraindications for bronchial provocation test were: FEV1 <60% predicted (adults or children) or <1.5 L (adults), inability to perform acceptable and repeatable spirometry maneuvers throughout the test procedure, myocardial infarction or stroke in the last 3 months, uncontrolled hypertension, known aortic aneurysm, recent eye surgery or intracranial pressure elevation risk, inability to perform any of the testing maneuvers, such as inhaling the challenge agent consistently [14].
FEV1 was measured at baseline and after inhalation of provocholine from 2mg/mL to 16.0mg/mL in normal saline solution. The various doses were delivered using a Respironics Philips nebulizer and inhaled by tidal breathing for 2 minutes with the nose clipped. FEV1 was measured at 30 seconds, 90 seconds, and 120 seconds after each dose. Doubling concentrations of provocholine were given at 5-minute intervals until the FEV1 decreased by 20% from baseline or until a dose of 16 mg/mL had been reached. Individuals with a decrease in FEV1 of 20% or more with 16mg/mL of provocholine or less, were defined as having airway hyperresponsiveness characteristic of current asthma.
Follow up
All participants had a follow-up for 3 months and underwent new spirometry (pre and post) and clinical examination (3rd study visit). During the follow-up period, we asked all the participants to record a diary with their everyday symptoms, peak flow monitoring, use of a reliever medication in case of symptoms (wheezing or cough night awakening, etc) and to contact their physician immediately if symptoms start to worsen.
According to our protocol, all subjects with positive pre-post bronchodilation and a positive brochoprovocation test are confirmed as an asthmatic group and continue their treatment for asthma according to GINA guidelines. In those with negative MCT, we withheld ICS and LABA or other asthma medications (eg LTRA). Participants with a negative initial bronchoprovocation test who presented asthma symptoms during the follow-up period repeated the methacholine challenge test.
Statistical analysis
Data were analyzed statistically using IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp. For all statistical analyses, a two-sided p - value less than 0.05 was considered significant. Descriptive statistical analysis was performed for demographics and clinical characteristics. Frequencies were calculated for categorical variables and presented as percentages. Mean and confidence interval was constructed for continuous variables.
Results
Table 1 shows the demographics, medical history main symptoms, and spirometric and methacholine test results of the three groups (Total number, confirmed, and Ruled-out). The mean age of the subjects was 47,8 (SD15,8) years, 29 were men (48,3%) and 31 were females (51,7%). All subjects were non-smokers according to the protocol. Of the 60 participants, 27 (45%) were confirmed to have asthma during the diagnostic assessment period. The diagnosis was confirmed in 16 (26.6%), by the reversibility test in pre and post-spirometry after salbutamol administration at the 1st visit. At the second visit 9(15%) subjects were confirmed as asthmatics by demonstrating bronchial hyperresponsiveness via bronchial challenge testing. At the 3rd study visit (after the follow-up period of the 3 months ) two additional subjects (3.3%) who had the first MCT negative, during the 3 months follow-up period developed asthma symptoms. These two participants had a negative pre and post-bronchodilation test but a second bronchial challenge test was positive.. Thus, 33 (55%) subjects showed no evidence of reversibility airflow obstruction, bronchial hyperresponsiveness, or worsening of asthmatic symptoms (after discontinuation of asthma treatment with inhaled steroids and other asthma medications) during the 3 months observational period and were considered finally as non-asthmatics (ruled-out).
No statistically significant difference was found between confirmed and ruled-out groups concerning the demographics or the doctor’s specialty that made the initial diagnosis (Table 1).
In the ruled-out subjects of the study previously diagnosed as asthmatics, other clinical diagnoses were possible based on the history of their symptoms - like upper airway cough syndrome, allergic rhinitis, or gastroesophageal regurgitation (GER).
The total number of months that the participants received asthma treatment before the study, was estimated, multiplied by the number of devices that they used every month and their price to find the average annual total cost. .Furthermore. The cost of treatment in asthma ruled out the group was found approximately 297 euro/patient/year (179-415, 95% CI) and for asthma confirmed the group was 313 (171-454, 95% CI) euro/patient/year.
Discussion
The main finding of the study was that 33 out of 60 adult participants (55.1%), who had been previously diagnosed with asthma by physicians in Cyprus at outpatients private clinical practice and who had been under treatment with asthma medications ( inhaled steroids, LABA, LTRA, etc.) had no evidence of current asthma, when they were prospectively evaluated with a long term follow-up with serial assessments of respiratory symptoms and with specific lung function tests as pre and post bronchodilation test and specific bronchial provocation tests according to those suggested at current guidelines for asthma diagnosis
Our results might indicate overdiagnosis of asthma in our community at the outpatient clinical practice in Cyprus that needs to be taken into account for further better educational courses to highlight the need for the use of other more specific tests for diagnosis of asthma as is lung function tests - baseline spirometry with pre and post bronchodilatation but also with another specific test like bronchial provocation test. The spontaneous remission of previously active asthma during the study period (within the 3 months follow-up) according to our protocol is unlikely since the follow-up period was not in or out of season and was considered a relevant time for clinical follow-up that can be realistic at common clinical primary care practice in order to identify a relapse in subjects withholding asthma treatment.
Therefore, we may assume that over-diagnosis of asthma occurs in Cyprus in primary care at the outpatient clinical practical care level. In agreement with our results is the study that was published recently by Aaron, et al [15]. They reported that Asthma was ruled out in 33% of participants and after 12 months 30% remained without asthma medication. In that study of 613 participants, meth choline provocation was performed on all the participants. In our study, we repeated MCT only to the subjects that continued to exhibit asthma-like symptoms during the follow-up period and this is one of our study limitations.
Joyce, et al. [16] examined a heterogeneous referred sample of patients sent to pulmonologists for further evaluation by MCT. They reported that of the 175 patients with a negative Methacholine Challenge test defined as a PC20 of greater than 8.0 mg/mL, 74% had been previously labeled as asthmatics by their primary care physician. This study was similar to our study in that their sample was a referred population to pulmonologists for further evaluation.
Pratter, et al. [17] examined 34 patients referred with wheeze and found that a prior diagnosis of asthma was predictive of having asthma in only 62% of the sample. The similarity in methodology and design of this study with our study is that it investigates a population of patients who had been referred to a specialist (pulmonologist), in order to investigate the extent of possible over-diagnosis of asthma.
In addition, Linden, et al. [18] showed that 41% of a sample of physicians labeled asthmatics showed no evidence of reversible airflow obstruction and had a negative methacholine challenge. In that study they found that only 52.2% of the subjects reported that they had ever undergone pulmonary function testing, similarly, we found a respective 46,6%.
Therefore, most of the studies with similar protocols to ours had shown results indicating an overdiagnosis of asthma.
Among the physicians that initially made the asthma misdiagnosis 57.6% of the cases were pulmonologists, 24.2% were internists and 12.1% were general practitioners.
According to our study, there may be consequences associated with over-treating asthma in a significant number of the ruled - out subjects (55%) Although inhaled steroids as medications for asthma treatment are generally safe, the use of any asthma medication among overdiagnosed patients without asthma is not needed and indicates the need for better evaluation of patients with respiratory symptoms to define asthma or another diagnosis that may mimic asthma symptoms and chose the proper treatment [19] as “wheezing “or “coughing” is not always asthma. Consequences also include the patient’s potential exposure to the adverse effects of asthma medications [20-21] and the costs of asthma medications. These patients are also incurring additional healthcare expenditures without therapeutic benefit.
The average cost of asthma medication for both groups was 304 euros/patient (216-393, 95% CI) for the period of 2 years before the study. This was similar to the 207,97 euros of the annual cost for asthma medications reported by Zannetos S, et al. [22]. Furthermore, the participants of the “asthma ruled out group” may have scheduled a similar number of annual primary care or specialist care visits for routine monitoring of “asthma” activity or to fill new prescriptions. If other health conditions were responsible for the over-diagnosis of asthma, the costs may be different and the proper management of the underlying condition could confer further cost benefits.
Our results suggest that additional screening to correct an overdiagnosis of asthma would save asthma-related costs from the first time of diagnosis, with savings compounding in subsequent years. Savings could be reallocated to the management of individuals with a confirmed diagnosis of asthma, especially those with severe asthma who could pursue novel but expensive treatments or identify and treat the underlying diseases of over - diagnosed patients [23,24].
Based on clinical history we could better identify common alternative diagnoses such as upper airway cough syndrome, allergic rhinitis and gastroesophageal regurgitation.
One of the strengths of this study is that we used a population-based sample of patients with a physician diagnosis of asthma, including pulmonologists in the sample physicians meaning that our results are likely to be representative of routine healthcare use at the outpatients’ primary care clinic treating patients with asthma-like symptoms. Our study shows that even pulmonologists that didn’t follow the diagnostic algorithm (history, spirometry pre and post-bronchodilation, bronchial Challenge test) had a 57.6% false asthma diagnosis.
Our study has several limitations. Our estimates of overdiagnosis may be conservative due to the likelihood of false positive methacholine challenge tests [25]; this in turn may have resulted in an underestimation of cost. On the other side, our study cut point for positive MBC was PC20 16 ≤ mg/ml, and in most studies was PC20 ≤ 8 mg/ml. That difference means that the percentage of asthma ruled out by the group in our study could have been even higher if we had used the PC20 of 8mg/ml. ATS guidelines suggest a PC20 between 4mg/mL and 16mg/ml. There is a possibility that subjects with a negative PC20 would have subsequently had a positive PC20 if the 16mg/mL threshold was used for all subjects [26]. The reason that we decided to use the cut-off of 16mg/mL for PC20 is to reduce to a minimum the ‘’false’’ negative diagnoses.
We excluded participants in whom an objective diagnostic test was contraindicated due to asthma - related reasons, which may have resulted in a lower representation of participants with more severe disease and therefore higher resource utilization. The sensitivity of bronchial challenge tests to detect asthma is 98%, meaning that a very small number of individuals with current asthma may have been missed by the study testing algorithm. Conversely, the specificity of bronchial challenge tests is less than 80% and is well known that the test can be positive in patients with allergic rhinitis or might be influenced by smoking status. This is not the case in our study as we included only non -smokers. Allergic rhinitis might co-exist with asthma and might also affect the bronchial provocation test. Patients with allergic rhinitis may also have presented with asthma - like symptoms - most commonly with cough -thus further careful evaluation of these subjects is necessary to better define the right diagnosis and choose the right treatment and not to simplify the approach of the disease based only on clinical symptoms.
Furthermore, in our study, we did a simple estimation of the medication’s asthma - related costs as an indication of the possible cost of asthma treatment in our population but of course, this is only an indication. Since Cyprus is a small country (1.2 million population) the sample size was calculated in accordance with the current literature [16,17]. However .further studies in a larger group of subjects with a more specific detailed protocol need to be designed in the future for a proper assessment of the health care cost of a common respiratory disease -such as asthma and the relevant treatment.
Conclusions
This study was performed for the first time in Cyprus to define the diagnostic approach to asthma at an outpatient primary care practice level in the country.
Our study has shown that more than the 50% of patients diagnosed as asthmatics at primary care outpatient clinical practice, were ruled out of the diagnosis after objective diagnostic testing with specific lung function tests and a proper close 3months clinical follow-up. These results indicate an over-diagnosis of asthma in Cyprus and a consequent over - treatment with asthma medication. Our results suggest that whenever possible, physicians should order objective tests, such as pre - bronchodilator and post - bronchodilator spirometry, or bronchial challenge tests, to confirm asthma at the time of initial diagnosis.
According to this study, doctors have not to oversimplify the diagnosis of asthma, but to approach carefully the diagnosis of the disease according to international guidelines and have to use more specific tests for a better evaluation of the disease. Lung function with pre and post - spirometry but also other specific tests - like bronchial challenge test - are helpful in order to make a clear and proper diagnosis of the disease and prescribe the right treatment.
In addition, the benefits for the health care systems when asthma diagnosis is better confirmed - based on serial diagnostic algorithms - as is suggested by all the international guidelines-need to be further evaluated in the future for Cyprus after advanced educational programs in order to reduce the health cost of a common respiratory disease-as asthma with the relevant increased cost of treatment.
- Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, Cruz AA, Duijts L, Drazen JM, FitzGerald JM, Fleming LJ, Inoue H, Ko FW, Krishnan JA, Levy ML, Lin J, Mortimer K, Pitrez PM, Sheikh A, Yorgancioglu AA, Boulet LP. Global Initiative for Asthma Strategy 2021: Executive Summary and Rationale for Key Changes. J Allergy Clin Immunol Pract. 2022 Jan;10(1S):S1-S18. doi: 10.1016/j.jaip.2021.10.001. Epub 2021 Oct 28. PMID: 34718211.
- Bloom CI, Saglani S, Feary J, Jarvis D, Quint JK. Changing prevalence of current asthma and inhaled corticosteroid treatment in the UK: population-based cohort 2006-2016. Eur Respir J. 2019 Apr 4;53(4):1802130. doi: 10.1183/13993003.02130-2018. PMID: 30765507.
- To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, Boulet LP. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012 Mar 19;12:204. doi: 10.1186/1471-2458-12-204. Erratum in: BMC Public Health. 2021 Oct 8;21(1):1809. PMID: 22429515; PMCID: PMC3353191.
- Pearce N, Aït-Khaled N, Beasley R, Mallol J, Keil U, Mitchell E, Robertson C; ISAAC Phase Three Study Group. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. 2007 Sep;62(9):758-66. doi: 10.1136/thx.2006.070169. Epub 2007 May 15. PMID: 17504817; PMCID: PMC2117323.
- Lai CK, Beasley R, Crane J, Foliaki S, Shah J, Weiland S; International Study of Asthma and Allergies in Childhood Phase Three Study Group. Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. 2009 Jun;64(6):476-83. doi: 10.1136/thx.2008.106609. Epub 2009 Feb 22. PMID: 19237391.
- Mallol J, Crane J, von Mutius E, Odhiambo J, Keil U, Stewart A; ISAAC Phase Three Study Group. The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three: a global synthesis. Allergol Immunopathol (Madr). 2013 Mar-Apr;41(2):73-85. doi: 10.1016/j.aller.2012.03.001. Epub 2012 Jul 6. PMID: 22771150.
- Surendran Aneeshkumar, Singh Raj. Economic burden of asthma among patients visiting a private hospital in south India. 2017. https://doi.org/10.1183/1393003.congress-2017.PA2800
- Loftus PA, Wise SK. Epidemiology and economic burden of asthma. Int Forum Allergy Rhinol. 2015 Sep;5 Suppl 1:S7-10. doi: 10.1002/alr.21547. Epub 2015 May 23. PMID: 26010063.
- Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018 Feb 24;391(10122):783-800. doi: 10.1016/S0140-6736(17)33311-1. Epub 2017 Dec 19. PMID: 29273246.
- Kavanagh J, Jackson DJ, Kent BD. Over- and under-diagnosis in asthma. Breathe (Sheff). 2019 Mar;15(1):e20-e27. doi: 10.1183/20734735.0362-2018. PMID: 31031841; PMCID: PMC6481983.
- Hunter CJ, Brightling CE, Woltmann G, Wardlaw AJ, Pavord ID. A comparison of the validity of different diagnostic tests in adults with asthma. Chest. 2002 Apr;121(4):1051-7. doi: 10.1378/chest.121.4.1051. PMID: 11948032.
- Coates AL, Wanger J, Cockcroft DW, Culver BH; Bronchoprovocation Testing Task Force: Kai-Håkon Carlsen, Diamant Z, Gauvreau G, Hall GL, Hallstrand TS, Horvath I, de Jongh FHC, Joos G, Kaminsky DA, Laube BL, Leuppi JD, Sterk PJ. ERS technical standard on bronchial challenge testing: general considerations and performance of methacholine challenge tests. Eur Respir J. 2017 May 1;49(5):1601526. doi: 10.1183/13993003.01526-2016. PMID: 28461290.
- Aaron SD, Boulet LP, Reddel HK, Gershon AS. Underdiagnosis and Overdiagnosis of Asthma. Am J Respir Crit Care Med. 2018 Oct 15;198(8):1012-1020. doi: 10.1164/rccm.201804-0682CI. PMID: 29756989.
- Cooper BG. Republished review: An update on contraindications for lung function testing. Postgrad Med J. 2011 Oct;87(1032):724-33. doi: 10.1136/pgmj.2010.139881rep. PMID: 21954034.
- Aaron SD, Vandemheen KL, FitzGerald JM, Ainslie M, Gupta S, Lemière C, Field SK, McIvor RA, Hernandez P, Mayers I, Mulpuru S, Alvarez GG, Pakhale S, Mallick R, Boulet LP; Canadian Respiratory Research Network. Reevaluation of Diagnosis in Adults With Physician-Diagnosed Asthma. JAMA. 2017 Jan 17;317(3):269-279. doi: 10.1001/jama.2016.19627. PMID: 28114551.
- Joyce DP, Chapman KR, Kesten S. Prior diagnosis and treatment of patients with normal results of methacholine challenge and unexplained respiratory symptoms. Chest. 1996 Mar;109(3):697-701. doi: 10.1378/chest.109.3.697. PMID: 8617078.
- Pratter MR, Hingston DM, Irwin RS. Diagnosis of bronchial asthma by clinical evaluation. An unreliable method. Chest. 1983 Jul;84(1):42-7. doi: 10.1378/chest.84.1.42. PMID: 6861547.
- LindenSmith J, Morrison D, Deveau C, Hernandez P. Overdiagnosis of asthma in the community. Can Respir J. 2004 Mar;11(2):111-6. doi: 10.1155/2004/276493. PMID: 15045041.
- Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: A systematic review and meta-analysis. Arch Intern Med. 1999 May 10;159(9):941-55. doi: 10.1001/archinte.159.9.941. PMID: 10326936.
- Drazen JM, O'Byrne PM. Risks of long-acting beta-agonists in achieving asthma control. N Engl J Med. 2009 Apr 16;360(16):1671-2. doi: 10.1056/NEJMe0902057. PMID: 19369675.
- Molimard M, Girodet PO, Van Ganse E. Interest of pharmacoepidemiology for the study of inhaled drugs. Therapie. 2019 Apr;74(2):233-237. doi: 10.1016/j.therap.2018.08.002. Epub 2018 Oct 19. PMID: 30392699.
- Zannetos S, Zachariadou T, Zachariades A, Georgiou A, Talias MA. The economic burden of adult asthma in Cyprus; a prevalence-based cost of illness study. BMC Public Health. 2017 Mar 16;17(1):262. doi: 10.1186/s12889-017-4184-0. PMID: 28302094; PMCID: PMC5356320.
- Corrigan SP, Cecillon DL, Sin DD, Sharpe HM, Andrews EM, Cowie RL, Man SF. The costs of implementing the 1999 Canadian Asthma Consensus Guidelines recommendation of asthma education and spirometry for the family physician. Can Respir J. 2004 Jul-Aug;11(5):349-53. doi: 10.1155/2004/914865. PMID: 15332137.
- Sullivan PW, Ghushchyan VH, Slejko JF, Belozeroff V, Globe DR, Lin SL. The burden of adult asthma in the United States: evidence from the Medical Expenditure Panel Survey. J Allergy Clin Immunol. 2011 Feb;127(2):363-369.e1-3. doi: 10.1016/j.jaci.2010.10.042. Erratum in: J Allergy Clin Immunol. 2012 Feb;129(2):587-8. PMID: 21281868.
- Yurdakul AS, Dursun B, Canbakan S, Cakaloğlu A, Capan N. The assessment of validity of different asthma diagnostic tools in adults. J Asthma. 2005 Dec;42(10):843-6. doi: 10.1080/02770900500370981. PMID: 16393722.
- McGrath KW, Fahy JV. Negative methacholine challenge tests in subjects who report physician-diagnosed asthma. Clin Exp Allergy. 2011 Jan;41(1):46-51. doi: 10.1111/j.1365-2222.2010.03627.x. Epub 2010 Nov 24. PMID: 21105916; PMCID: PMC3059141.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
