Diet, Nutrition and Paediatric Asthma: Emerging Trends
1Department of Food and Nutrition Education, Faculty of Health, Allied Sciences and Home Economic Education, University of Education, Winneba, Ghana
2Department of Integrated Home Economics Education, Faculty of Health, Allied Sciences and Home Economics Education, University of Education, Winneba, Ghana
Author and article information
Cite this as
Anyimah-Ackah E, Amfo-Antiri A, Eshun G. Diet, Nutrition and Paediatric Asthma: Emerging Trends. Open J Asthma. 2025; 9(1): 001-007. Available from: 10.17352/oja.000021
Copyright License
© 2025 Anyimah-Ackah E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: Childhood asthma keeps rising, and what lands on the dinner table may stoke or soothe young airways. Understanding the dietary influences shaping this epidemic is both a public health priority and a culinary question.
Objective: This scoping review charts the past decade of research exploring how whole diets, individual nutrients, and body weight affect asthma in children while highlighting culturally grounded dietary interventions.
Methods: We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Scoping Review guidance. Searches of eight databases and gray literature captured observational and experimental studies published from January 2015 to May 2025 on diet and asthma in individuals from birth to eighteen years. Two reviewers screened, extracted, and assessed quality.
Results: Plant-rich patterns, including Mediterranean and Dietary Approaches to Stop Hypertension (DASH) menus, were associated with fifteen to fifty percent fewer wheezing episodes, while fast food, sugar-sweetened drinks, and other ultra-processed foods coincided with more attacks and hospital visits. Six trials that boosted fruits, vegetables, fish, or weight loss improved symptoms within six months; high-dose vitamin D and other single-nutrient pills rarely helped.
Conclusion: Diet quality and body weight are modifiable determinants of paediatric asthma worldwide. Clinicians should incorporate nutrition and weight counselling into asthma management, and public health policies that promote affordable, minimally processed foods may lessen disease burden. Future multicentre trials should test comprehensive dietary interventions and clarify microbiome-mediated mechanisms. Standardised outcome sets will enhance comparability across future nutritional trials worldwide and registries.
Asthma remains one of the most common chronic diseases in childhood, currently affecting ~300 million people worldwide, including at least 30 million children—a figure that continues to rise, particularly in rapidly modernising regions [1]. While traditional risk factors such as allergens, air pollution, and genetics are well recognised, shifts in lifestyle—especially diet—have received growing attention [1,2]. Over the past two decades, many populations have transitioned from traditional, largely plant-based diets to so-called “Western” diets characterised by highly processed foods, excess saturated and omega-6 fats, and sugar-sweetened beverages [2]. These dietary changes track closely with escalating rates of obesity and non-communicable diseases and now appear to parallel childhood asthma trends as well.
A biological link between diet and asthma is highly plausible. Asthma is fundamentally an inflammatory airway disorder; nutrients can amplify or quell that inflammation. Diets rich in antioxidants (fruits, vegetables, whole grains) and anti-inflammatory fats (omega-3s from fish) may counter oxidative stress and modulate immune responses, whereas diets high in saturated fats or excessive omega-6 fatty acids can promote a pro-inflammatory milieu [2]. Observational evidence supports these mechanisms: children adhering to Mediterranean- or Dietary Approaches to Stop Hypertension diet (DASH)-style patterns consistently show lower odds of wheeze and asthma, while high fast-food intake predicts more severe symptoms and emergency visits.
Interventional data, although fewer, lend additional weight. Trials of targeted weight loss in obese children improve symptom control, and six-month fruit-and-vegetable enrichment has demonstrated favourable trends in lung function and systemic inflammation, albeit without large reductions in exacerbations [1]. Collectively, these findings suggest that diet quality is a modifiable factor in both the prevention and management of paediatric asthma.
Importantly, dietary recommendations cannot be one-size-fits-all. Culinary traditions, food availability, and sociocultural beliefs shape eating habits and must be considered when designing effective interventions, particularly in low- and middle-income countries where nutrition transition intersects with unique environmental exposures and healthcare challenges. This review, therefore, synthesises evidence published between 2015 and 2025 on dietary patterns, specific nutrients, and nutritional status about childhood asthma, evaluates biological mechanisms, and highlights the need for culturally tailored, guideline-concordant strategies to reduce the global burden of paediatric asthma.
Dietary patterns and key nutrients – observational evidence
Asthma-related nutrition research (Table 1) over the last decade points to overall diet quality rather than single foods as the clearest dietary signal. Two contrasting patterns dominate.
Mediterranean-/DASH-style (“healthy”) patterns
Across Latin America, the Middle East, and the Pacific, higher adherence to fruit-, vegetable-, whole-grain-, and fish-rich diets consistently predicts lower asthma prevalence or milder symptoms. Examples include a Peruvian case–control study in which high Mediterranean-diet scores halve the odds of childhood asthma, and an Iranian adolescent survey where top-quartile DASH adherence cut doctor-diagnosed asthma by almost 50 % [3,6]. A 2023 meta-analysis pooling 65 paediatric studies (≈560,000 participants) estimated a 15 % reduction in current asthma for “healthy” dietary patterns overall [1].
Western / ultra-processed patterns
Fast-food–heavy, high-sugar, high-saturated-fat diets show the opposite trend. Latin-American International Study of Asthma and Allergies in Childhood (ISAAC) cohorts found ≥ 3 fast-food meals per week raised severe wheeze by ~40% in teenagers, while an Iranian survey linked Western-diet scores to greater wheeze in boys even after socioeconomic adjustment [4,5]. In a Puerto-Rican cohort followed for five years, children who persisted with poor-quality diets tripled their risk of incident asthma and doubled severe exacerbations [7].
Emerging evidence and cultural context
Plant-based and traditional diets in Low- and Middle-Income Countries, LMICs, often mirror Mediterranean principles and may offer similar protection; conversely, rapid nutrition transition in Ghana, Nigeria, or Thailand is introducing Westernised snacks and beverages linked to rising asthma rates [8,9]. Effective interventions, therefore, need to translate core “healthy-pattern” elements—abundant seasonal produce, legumes, nuts, modest fish—into locally affordable dishes and culinary practices rather than importing foreign menus.
Food groups and bioactive nutrients
Fruits and vegetables supply antioxidants (vitamins C/E, carotenoids) that dampen airway oxidative stress; higher intake correlates with fewer wheeze episodes in multi-country analyses [4,10–12]. Fast foods and processed meats contribute saturated fat, sodium, and nitrite preservatives that aggravate inflammation and track with emergency-room visits in adolescents [4,5,13]. Whole grains and dietary fibre foster gut bacteria that release short-chain fatty acids (SCFAs) with systemic anti-inflammatory effects, a mechanism gaining support in paediatric cohorts [1,14,15]. Concerning omega-3 versus omega-6 fatty acids, higher fish-derived EPA (Eicosapentaenoic acid) and DHA (Docosahexaenoic acid) intake is associated with fewer symptom days, while excess omega-6 from processed vegetable oils aligns with pollution-induced neutrophilic inflammation in urban U.S. children [16].
Low serum 25-hydroxyvitamin D, 25(OH)D, frequently co-occurs with poor asthma control, yet mega-dose supplementation has shown inconsistent benefits, underscoring the value of diet-first sufficiency rather than high-dose pills [17-20]. Cross-sectional U.S. data, among others, reveal lower odds of asthma in children with high composite antioxidant intake, and a Thai Randomized Controlled Trial (RCT) adding tomato- and mixed-fruit juices improved Asthma Control Test scores over eight weeks [4,10-12].
Diet-based asthma interventions
When children are nudged back toward real food—think bright fruit, crunchy vegetables, oily fish rather than foil-wrapped snacks—small but tangible breaths of relief follow. In Australia, boosting children’s plates to seven daily servings of produce raised plasma carotenoids and eased airway resistance, even if full-blown attacks held steady [21]. In Thailand, a short eight-week ritual of tomato-and-mixed-fruit juice nudged Asthma Control Test scores upward and brightened quality of life [10]. Yet when nutrition is distilled into single pills, the story grows murkier: neither high-dose vitamin D₃ for Indian school-children [19] nor a year-long U.S. trial of 4,000 IU daily [22] moved the needle on flare-ups. Timing, however, may be everything—prenatal fish oil offered to Danish mothers trimmed their offspring’s asthma risk by nearly a third [23]. And where excess weight presses on lungs like a too-tight belt, diet-and-movement programmes in the Netherlands [24] and Australia [25] lightened both Body Mass Index (BMI) z-scores and symptom burdens. Taken together, the trials whisper a Pollan-esque lesson: eat food—real, varied, mostly plants—begin early, keep moving, and reserve megadose capsules for clearly demonstrated gaps (Table 2).
Childhood diet-asthma mechanistic pathways
Asthma is driven by chronic airway inflammation, oxidative stress, and immune dysregulation; diet can reinforce or interrupt each of these processes through multiple, overlapping routes (Table 3).
Cultural tailoring in practice
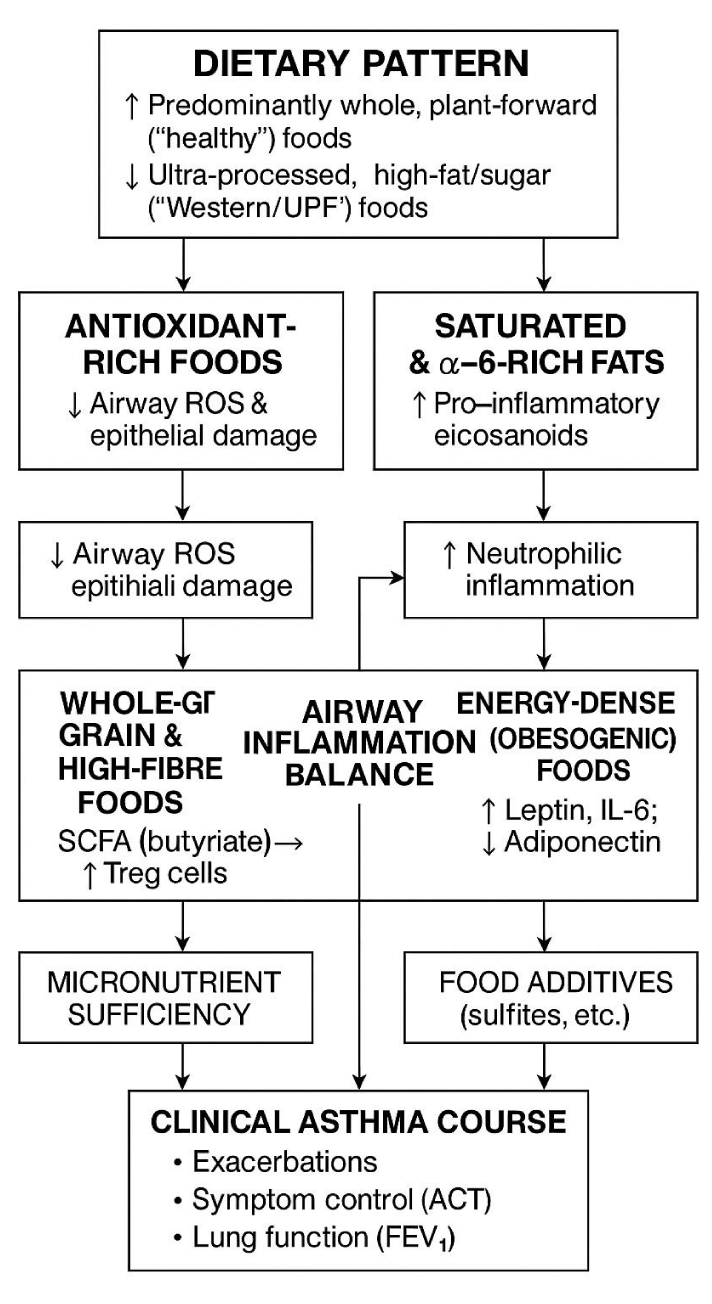
Core “anti-inflammatory” elements can be delivered through local cuisines: for instance, kontomire stew (leafy-green antioxidants), smoked sardines (omega-3), millet banku or waakye (whole-grain fibre), and ground-nut soups (plant protein, magnesium). Framing advice around familiar dishes improves adherence and equity, critical in Ghana and other LMICs undergoing rapid nutrition transition (Figure 1).
The diagram contrasts plant-forward, minimally processed diets (left stream) with ultra-processed, energy-dense patterns (right stream). Antioxidant-rich foods, whole-grains, and adequate micronutrients lower airway Reactive-oxygen Species (ROS), support Short-chain-fatty-acid (SCFA)–driven regulatory T-cell expansion, and preserve epithelial integrity—collectively tilting the “airway inflammation balance” toward resolution. In contrast, saturated/ω-6-rich fats, excess caloric load, and food additives amplify pro-inflammatory eicosanoids, neutrophilic infiltration, and systemic mediators (↑ leptin, IL-6; ↓ adiponectin), driving bronchoconstriction. The net interplay of these nodes determines clinical asthma course, reflected in exacerbation frequency, Asthma Control Test (ACT) scores, and lung function (FEV₁). Abbreviations: SCFA: Short-chain Fatty Acid; ROS: Reactive Oxygen Species; Treg: Regulatory T Cell; ACT: Asthma Control Test; FEV₁: Forced Expiratory Volume in one second.
Clinical implications and alignment with current guidelines
Why diet now belongs in every asthma review
The 2025 Global Initiative for Asthma (GINA) pocket guide and the 2024 National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) Expert Panel Report 4 (EPR-4) update both underline weight control, trigger avoidance and treatment adherence as cornerstones of paediatric asthma care [31,32]. Neither guideline yet issues formal dietary prescriptions, but both encourage attention to “modifiable lifestyle factors”—a door newly opened by the surge of evidence reviewed here. Observational and interventional data show that upgrading overall diet quality (and, where needed, reducing excess weight) can have clinical advantages. It improves Asthma Control Test (ACT) or Childhood-ACT scores by ~1 point within 2–6 months [21]. It enhances bronchodilator responsiveness in overweight phenotypes [24]. It modestly extends the interval between severe exacerbations when combined with guideline-step pharmacotherapy [22].
Practical steps for the clinic
(Table 4)
Translating evidence into culturally tailored care
Global guidelines urge context-specific implementation: In Ghana and similar settings, leverage local staples to effect. Millet porridge, ground-nut soups, and waakye deliver whole-grain fibre, magnesium, and plant protein that fit anti-inflammatory targets [33].
Use community assets: School feeding programmes, church cook-outs, and urban gardens can embed guideline-concordant meals into daily life.
Address affordability: Seasonal produce (for instance, fruits and vegetables including mango, orange, and kontomire) often costs less than imported snacks but meets antioxidant goals. Such tailoring aligns with the equity emphasis in both GINA and NIH EPR-4, ensuring dietary advice is actionable for diverse families [31,32].
Key messages for stakeholders
Clinicians may have to add quick diet checks to annual reviews; partner with dietitians for high-risk children. Public-health planners should embed nutrition education in asthma outreach and integrate produce subsidies or junk-food levies to reduce disparities [31]. Researchers must prioritise long-duration, culturally adapted diet trials that report guideline-relevant endpoints (exacerbation rate, ACT, FEV₁). Taken together, current evidence supports moving diet from the margins to the mainstream of paediatric asthma management—complementing, not replacing, pharmacotherapy, and delivered in culturally authentic ways that resonate with children and caregivers.
Discussion and future directions
This review confirms that diet quality is a clinically relevant, modifiable factor in paediatric asthma. Healthy dietary patterns—characterised by abundant fruits, vegetables, whole grains, lean proteins, and omega-3–rich seafoods-are consistently associated with lower asthma prevalence, milder symptoms, and better lung function [34,35]. Conversely, energy-dense, ultra-processed diets track with worse control and more frequent exacerbations, independent of adiposity [36].
Several observations stand out. First, overall dietary pattern explains more variance in outcomes than individual nutrients [34]. This supports a shift from supplement-centric advice (e.g., high-dose vitamin D or fish-oil capsules) toward whole-food approaches that deliver synergistic combinations of antioxidants, fibre, and anti-inflammatory lipids [2]. Second, beneficial effects appear strongest in children who are either nutrient-deficient (low serum 25-hydroxy-vitamin D, scant fruit-and-vegetable intake) or overweight/obese, suggesting that diet acts as both an anti-inflammatory and weight-modulating therapy [37]. Third, intervention trials—though still heterogeneous and often small—demonstrate that even modest dietary upgrades can improve symptom scores within months, with negligible adverse effects.
These findings dovetail with current GINA and NIH/NHLBI recommendations that clinicians address “modifiable lifestyle factors.” Incorporating a brief, culturally attuned diet screen into routine reviews can identify children likely to benefit from dietitian referral or structured weight management. In resource-constrained settings such as in Ghana, leveraging culturally familiar dishes—kontomire stew, millet porridge, smoked sardines—offers a feasible path to an anti-inflammatory diet without inflating household food costs.
Most observational studies are cross-sectional, limiting causal inference and vulnerable to reverse causation (children with poorer control may avoid exercise and gravitate toward convenience foods). Many trials rely on self-reported intake and lack blinding, increasing bias. Heterogeneity in outcome definitions (wheeze vs. asthma, ACT vs. ACQ) also hinders pooling. Finally, few studies examine long-term sustainability or implementation in LMICs where nutrition transition is rapidly underway.
Future research priorities include longer, adequately powered RCTs comparing culturally tailored ‘whole-diet’ interventions with usual care, and reporting guideline-relevant endpoints such as severe exacerbation rates and lung function trajectories. Pragmatic implementation studies are also necessary to embed diet counselling into national asthma programmes and school-feeding schemes, particularly in LMICs. Mechanistic research should integrate metabolomics, gut microbiome profiling, and airway inflammatory markers to clarify how specific food components such as short-chain fatty acids and carotenoids modulate immune pathways [38]. In addition, equity-focused cost-effectiveness analyses are important to evaluate whether produce subsidies or junk food levies reduce asthma morbidity in socioeconomically disadvantaged communities.
While awaiting definitive trials, clinicians can safely recommend: (i) increasing intake of diverse, locally available fruits and vegetables; (ii) swapping ultra-processed snacks for whole-grain staples [20]; (iii) ensuring adequate—but not pharmacologic—vitamin D and omega-3 status; and (iv) pursuing gradual, family-based weight management in children with obesity. These actions align with pharmacological management steps and pose minimal risk, making diet modification a pragmatic “add-on” to guideline-directed therapy.
- Zhang J, He M, Yu Q, Xiao F, Zhang Y, Liang C, et al. The Effects of a Healthy Diet on Asthma and Wheezing in Children and Adolescents: A Systematic Review and Meta-Analysis. J Asthma Allergy. 2023;16:1007–24. Available from: https://doi.org/10.2147/JAA.S423884
- Okoniewski W, Lu KD, Forno E. Weight Loss for Children and Adults with Obesity and Asthma. A Systematic Review of Randomized Controlled Trials. Ann Am Thorac Soc. 2019;16(5):613–25. Available from: https://doi.org/10.1513/AnnalsATS.201810-651SR
- Rice JL, Romero KM, Galvez Davila RM, Meza CT, Bilderback A, Williams DL, et al. Association Between Adherence to the Mediterranean Diet and Asthma in Peruvian Children. Lung. 2015;193(6):893–9. Available from: https://doi.org/10.1007/s00408-015-9792-9
- Cepeda AM, Thawer S, Boyle RJ, Villalba S, Jaller R, Tapias E, et al. Diet and Respiratory Health in Children from 11 Latin American Countries: Evidence from ISAAC Phase III. Lung. 2017;195(6):683–92. Available from: https://doi.org/10.1007/s00408-017-0044-z
- Emrani AS, Sasanfar B, Jowshan MR, Behniafard N, Nafei Z, Salehi-Abargouei A. Association between a western diet and asthma among children and adolescents. Sci Rep. 2024;14(1):13240. Available from: https://doi.org/10.1038/s41598-024-64008-5
- Arabi V, Sasanfar B, Toorang F, Nafei Z, Behniafard N, Salehi-Abargouei A. Association between DASH diet and asthma symptoms among a large sample of adolescents: a cross-sectional study. BMC Nutr. 2024;10(1):92. Available from: https://doi.org/10.1186/s40795-024-00884-4
- Reyes-Angel J, Han YY, Rosser F, Forno E, Acosta-Pérez E, Canino G, et al. Diet, Asthma, and Severe Asthma Exacerbations in a Prospective Study of Puerto Rican Youth. J Allergy Clin Immunol Pract. 2022;10(4):1013-1019.e1. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2213219822000812
- Nel JH, Steyn NP. The Nutrition Transition and the Double Burden of Malnutrition in Sub-Saharan African Countries: How Do These Countries Compare with the Recommended LANCET COMMISSION Global Diet? Int J Environ Res Public Health. 2022;19(24):16791. Available from: https://doi.org/10.3390/ijerph192416791
- Mortimer K, Reddel HK, Pitrez PM, Bateman ED. Asthma management in low and middle-income countries: case for change. Eur Respir J. 2022;60(3):2103179. Available from: https://doi.org/10.1183/13993003.03179-2021
- Songnuy T, Ninla-aesong P, Thairach P, Thok-Ngaen J. Effectiveness of an antioxidant-rich diet on childhood asthma outcomes: A randomized controlled trial. BMC Nutr. 2025;11(1):89. Available from: https://doi.org/10.1186/s40795-025-01078-2
- Lin W, Lin J, Lai F, Shi J. Effect of dietary antioxidant quality score on tobacco smoke exposure and asthma in children and adolescents: a cross-sectional study from the NHANES database. BMC Pediatr. 2024;24(1):535. Available from: https://doi.org/10.1186/s12887-024-05009-1
- Hosseini B, Berthon BS, Wark P, Wood LG. Effects of Fruit and Vegetable Consumption on Risk of Asthma, Wheezing and Immune Responses: A Systematic Review and Meta-Analysis. Nutrients. 2017;9(4):341. Available from: https://doi.org/10.3390/nu9040341
- Valle K, Marcela E, Asa B, Paul B, Emanuel A, Cisneros R. Fast-food consumption and asthma-related emergency room visits in California. J Asthma. 2025;62(4):647–54. Available from: https://doi.org/10.1080/02770903.2024.2429679
- Verstegen REM, Kostadinova AI, Merenciana Z, Garssen J, Folkerts G, Hendriks RW, et al. Dietary Fibers: Effects, Underlying Mechanisms and Possible Role in Allergic Asthma Management. Nutrients. 2021;13(11):4153. Available from: https://www.mdpi.com/2072-6643/13/11/4153
- Andrianasolo RM, Hercberg S, Kesse-Guyot E, Druesne-Pecollo N, Touvier M, Galan P, et al. Association between dietary fibre intake and asthma (symptoms and control): results from the French national e-cohort NutriNet-Santé. Br J Nutr. 2019;122(9):1040–51. Available from: https://doi.org/10.1017/S0007114519001843
- Brigham EP, Woo H, McCormack M, Rice J, Koehler K, Vulcain T, et al. Omega-3 and Omega-6 Intake Modifies Asthma Severity and Response to Indoor Air Pollution in Children. Am J Respir Crit Care Med. 2019;199(12):1478–86. Available from: https://doi.org/10.1164/rccm.201808-1474OC
- Sung M. Trends of vitamin D in asthma in the pediatric population for two decades: a systematic review. Clin Exp Pediatr. 2023;66(8):339–47. Available from: https://doi.org/10.3345/cep.2022.01109
- Forno E, Bacharier LB, Phipatanakul W, Guilbert TW, Cabana MD, Ross K, et al. Effect of Vitamin D3 Supplementation on Severe Asthma Exacerbations in Children With Asthma and Low Vitamin D Levels. JAMA. 2020;324(8):752. Available from: https://jamanetwork.com/journals/jama/fullarticle/2769724
- Kumar J, Kumar P, Goyal JP, Thakur C, Choudhary P, Meena J, et al. Vitamin D supplementation in childhood asthma: a systematic review and meta-analysis of randomised controlled trials. ERJ Open Res. 2022;8(1):662–2021. Available from: https://doi.org/10.1183/23120541.00662-2021
- Fedora K, Asih SR, Qorri' A, Nur RL, Nural TN, Titiharja FF. Vitamin D supplementation decreases asthma exacerbations in children: a systematic review and meta-analysis of randomized controlled trials. Ann Med. 2024;56(1):2400313. Available from: https://doi.org/10.1080/07853890.2024.2400313
- Berthon BS, McLoughlin RF, Jensen ME, Hosseini B, Williams EJ, Baines KJ, et al. The effects of increasing fruit and vegetable intake in children with asthma: A randomized controlled trial. Clin Exp Allergy. 2021;51(9):1144–56. Available from: https://doi.org/10.1111/cea.13979
- Forno E, Bacharier LB, Phipatanakul W, Guilbert TW, Cabana MD, Ross K, et al. Effect of Vitamin D3 Supplementation on Severe Asthma Exacerbations in Children With Asthma and Low Vitamin D Levels: The VDKA Randomized Clinical Trial. JAMA. 2020;324(8):752–60. Available from: https://doi.org/10.1001/jama.2020.12384
- Bisgaard H, Stokholm J, Chawes BL, Vissing NH, Bjarnadóttir E, Schoos AMM, et al. Fish Oil–Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N Engl J Med. 2016;375(26):2530–9. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1503734
- Willeboordse M, Kant KDG van de, Tan FES, Mulkens S, Schellings J, Crijns Y, et al. A Multifactorial Weight Reduction Programme for Children with Overweight and Asthma: A Randomized Controlled Trial. PLoS One. 2016;11(6):e0157158. Available from: https://doi.org/10.1371/journal.pone.0157158
- Eslick S, Jensen ME, Collins CE, Gibson PG, Hilton J, Wood LG. Characterising a Weight Loss Intervention in Obese Asthmatic Children. Nutrients. 2020;12(2):507. Available from: https://doi.org/10.3390/nu12020507
- Thakur C, Kumar J, Kumar P, Goyal JP, Singh K, Gupta A. Vitamin-D supplementation as an adjunct to standard treatment of asthma in children: A randomized controlled trial (ViDASTA Trial). Pediatr Pulmonol. 2021;56(6):1427–33. Available from: https://doi.org/10.1002/ppul.25287
- Rostampour K, Sasanfar B, Reshadfar A, Emarati A, Nafei Z, Behniafard N, et al. The association between fruit and vegetable intake and the odds of asthma among children and adolescents. J Heal Popul Nutr. 2025;44(1):99. Available from: https://doi.org/10.1186/s41043-025-00820-7
- Boulund U, Thorsen J, Trivedi U, Tranæs K, Jiang J, Shah SA, et al. The role of the early-life gut microbiome in childhood asthma. Gut Microbes. 2025;17(1):2457489. Available from: https://doi.org/10.1080/19490976.2025.2457489
- Farhangi MA, Doumat G, Baroni IF, Camargo Jr. CA. Weight loss and asthma control: A systematic review of randomized controlled trials. Obes Rev. 2025;26(7):e13907. Available from: https://doi.org/10.1111/obr.13907
- Gupta MK, Basavaraj GV. Sulphites in food & drinks in asthmatic adults & children: What we need to know. Indian J Allergy, Asthma Immunol. 2021;35(2). Available from: https://journals.lww.com/ijaa/fulltext/2021/35020/sulphites_in_food___drinks_in_asthmatic_adults__.2.aspx
- GINA. Global strategy for asthma management and prevention. Global Initiative for Asthma; 2025. Available from: http://www.ginasthma.org/
- NHLBI. 2020 Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. 2020. Report No.: 20-HL-8140. Available from: https://www.nhlbi.nih.gov/sites/default/files/publications/AsthmaManagementGuidelinesReport-2-4-21.pdf
- Amoah S, Ennin R, Sagoe K, Steinbrecher A, Pischon T, Mockenhaupt FP, et al. Feasibility of a Culturally Adapted Dietary Weight-Loss Intervention among Ghanaian Migrants in Berlin, Germany: The ADAPT Study. Int J Environ Res Public Health. 2021;18(2):510. Available from: https://doi.org/10.3390/ijerph18020510
- Masini A, Dallolio L, Sanmarchi F, Lovecchio F, Falato M, Longobucco Y, et al. Adherence to the Mediterranean Diet in Children and Adolescents and Association with Multiple Outcomes: An Umbrella Review. Healthcare (Basel). 2024;12(4):449. Available from: https://doi.org/10.3390/healthcare12040449
- Ayats-Vidal R, Albiciuc IA, Bruch-Molist C, Cuartero-Gorjón A, Cordobilla B, Pedrosa-Domínguez M, et al. Erythrocyte Fatty Acid Profile, Mediterranean Diet and Asthma Severity in Childhood Allergic Asthma: Preliminary Findings from a Cohort Study in Spain. Nutrients. 2025;17:1161. Available from: https://doi.org/10.3390/nu17071161
- Zhou W, Tang J. Prevalence and risk factors for childhood asthma: a systematic review and meta-analysis. BMC Pediatr. 2025;25(1):50. Available from: https://doi.org/10.1186/s12887-025-05409-x
- Spriet SW, Davis KL. Diet-Induced Weight Loss in Obese Children With Asthma: A Randomized Controlled Trial. Pediatrics. 2014;134(Supplement_3):S170–S170. Available from: https://doi.org/10.1542/peds.2014-1817KKK
- Hsu CY, Khachatryan LG, Younis NK, Mustafa MA, Ahmad N, Athab ZH, et al. Microbiota-derived short chain fatty acids in pediatric health and diseases: from gut development to neuroprotection. Front Microbiol. 2024;Volume 15-2024. Available from: https://doi.org/10.3389/fmicb.2024.1456793
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
