Open Journal of Orthopedics and Rheumatology
Herbal Remedies as Adjunct Therapies for Rheumatoid Arthritis: Focus on Ginger: A Review
Judy Adham*
STEM High School, Giza, Egypt
Cite this as
Adham J. Herbal Remedies as Adjunct Therapies for Rheumatoid Arthritis: Focus on Ginger: A Review. Open J Orthop Rheumatol. 2025;10(1):021-025. Available from: 10.17352/ojor.000052Copyright License
© 2025 Adham J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Rheumatoid Arthritis (RA) is a chronic autoimmune disorder characterized by persistent joint inflammation, pain, and progressive disability. While conventional pharmacological treatments remain the cornerstone of RA management, growing interest in alternative and complementary therapies has drawn attention to the medicinal potential of natural compounds such as ginger (Zingiber officinale). This review investigates the therapeutic role of ginger in alleviating RA-related symptoms, with a particular focus on its anti-inflammatory and immunomodulatory mechanisms. Bioactive constituents such as gingerols and shogaols have demonstrated the ability to inhibit pro-inflammatory cytokines and oxidative stress pathways implicated in RA pathogenesis. Clinical and preclinical studies provide preliminary evidence supporting ginger's efficacy and safety. This paper highlights current findings and outlines future directions for integrating ginger as a complementary therapeutic option in RA management.
Introduction
Rheumatoid Arthritis (RA) is a chronic, systemic autoimmune disorder in which the immune system mistakenly attacks the synovial lining of joints, leading to persistent inflammation, pain, and joint destruction. In severe cases, this process may result in irreversible joint damage, functional disability, and diminished quality of life [1]. Beyond the musculoskeletal system, RA can affect extra-articular organs, including the lungs, heart, skin, eyes, and vasculature [1,2]. The global prevalence of RA is approximately 0.5%, with women affected two to three times more frequently than men. Peak onset typically occurs between 50 and 59 years of age [1,3].
The pathogenesis of RA is multifactorial and remains incompletely understood, involving genetic predisposition, environmental triggers, and dysregulated immune responses [4]. Its heterogeneous clinical presentation and variable therapeutic responses highlight the complexity of the disease [4,5]. RA is associated with increased risks of serious infections, interstitial lung disease, cardiovascular complications, malignancies, osteoporosis, and premature mortality compared to the general population [3,5]. Nevertheless, early diagnosis and aggressive treatment—particularly with disease-modifying antirheumatic drugs (DMARDs)—have significantly improved patient outcomes [3].
Clinically, RA often presents as symmetrical polyarthritis, primarily affecting the small joints of the hands, wrists, feet, and knees [1,6]. Morning stiffness lasting longer than 30 minutes, along with joint swelling, warmth, and tenderness, are hallmark symptoms [6]. In some cases, monoarthritis or oligoarthritis may precede more widespread involvement. Extra-articular manifestations, such as interstitial lung disease, pericarditis, and pleural effusion, may even occur without prominent joint symptoms [3]. Additional systemic symptoms include fatigue, low-grade fever, poor appetite, and unintended weight loss [6]. Despite advances in treatment, a subset of patients fails to achieve remission or low disease activity due to factors such as autoantibody positivity, joint erosion, genetic susceptibility, extra-articular involvement, or comorbid conditions [4,5].
The use of complementary and alternative medicine (CAM) has grown significantly among patients with chronic inflammatory conditions. In the United States, CAM utilization rose from 34% in 1990 to over 42% in 1997, surpassing even the frequency of primary care visits [7]. Herbal medicine—one of the most widely used CAM modalities—relies on various plant parts, often used synergistically, under the belief that whole plant formulations offer a more balanced and sustained healing effect compared to isolated compounds [8]. Among these, ginger (Zingiber officinale) has emerged as a promising adjunctive therapy due to its well-documented anti-inflammatory and immunomodulatory properties [8-10]. This review explores the current evidence regarding ginger's potential role as an adjunct treatment for rheumatoid arthritis [11-15].
Pathophysiology of rheumatoid arthritis
Rheumatoid Arthritis (RA) is a chronic autoimmune disease characterized by persistent inflammation of the synovial joints, leading to cartilage degradation, bone erosion, and functional impairment [16]. The pathogenesis involves a complex interplay between genetic predisposition and environmental triggers, initiating an aberrant immune response [4,17]. At the cellular level, the disease is marked by the infiltration of immune cells—such as T lymphocytes, B cells, macrophages, and dendritic cells—into the synovial tissue. These cells produce pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6), which drive the inflammatory cascade [18,19].
Additionally, the activation of nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinases (MAPKs) further amplifies the inflammatory response [19,20]. The synovial lining becomes hyperplastic, forming pannus tissue that invades and destroys adjacent cartilage and bone. This process is facilitated by the release of matrix metalloproteinases (MMPs) and receptor activator of nuclear factor-kappa B ligand (RANKL), which promote osteoclastogenesis and subsequent bone resorption [19,21].
Systemic manifestations of RA include fatigue, anemia, and an increased risk of cardiovascular disease [3,16]. The chronic inflammation can also affect other organs, leading to complications such as rheumatoid nodules, interstitial lung disease, and vasculitis [3]. Understanding the pathophysiological mechanisms of RA is crucial for developing targeted therapies aimed at modulating the immune response and preventing joint damage [17].
Inactive compounds in ginger
Ginger (Zingiber officinale Roscoe) is a member of the Zingiberaceae family of plants. Some studies have proven that ginger is the most-used herbal drug in many countries [22]. Scientific evidence supports its antioxidant and anti-inflammatory capacities; in contrast, a more specific and less-studied bioactivity is its possible neuroprotective effect [22,23]. Zingiber officinale is among the medicinal plants with beneficial health effects that have been widely used in both pharmaceutical products and food [8]. Its crude extract is known for its pharmacological effects, especially due to its bioactive phytochemicals [9,23].
Ginger rhizome is commonly added to food as a spice or taken as a dietary supplement, and has been widely used in traditional medicine across various cultures [8,24]. One of its main traditional uses includes treating urinary tract inflammatory problems [24]. Furthermore, its anti-inflammatory properties, due to immune response modulation during the cellular phase, have been well described [23].
Another highlighted effect of ginger extract is its antinociceptive activity induced by acetic acid [25]. Its bioactive compounds (e.g., gingerols and shogaols) have analgesic and anti-inflammatory properties by inhibiting the COX-2 and LOX pathways, thereby preventing arachidonic acid metabolism [23,25]. The action of ginger has been shown to resemble that of NSAIDs, though notably, it does not damage the gastric mucosa [26].
The rhizome of Zingiber officinale is extensively used in medicinal applications. Ayurvedic literature highlights its use for both communicable and non-communicable diseases [24]. Modern studies in analytical chemistry, cytology, and microbiology support its application in various disease conditions. Ginger is reported to have antiviral, radioprotective, anticancer, antioxidant, and anti-inflammatory effects, aligning with its Ayurvedic profile [24].
It is also considered beneficial for boosting appetite, immunity, and reinvigorating weakened physiological systems. Active constituents such as 6-gingerol, 6-shogaol, 6-paradol, zingerone, and zerumbone are believed to promote enzyme activity, regulate circulation, and rejuvenate the body by restoring physical strength [24].
Anti-inflammatory and immunomodulatory effects of ginger
Zingiber officinale is highly effective in treating inflammation associated with the alimentary tract, including conditions such as colitis [27]. Ginger’s bioactivity has been linked to its modulation of the phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt) pathway and the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway [28,29]. In particular, 6-shogaol has demonstrated protective effects against TNF-α-induced intestinal dysfunction in human intestinal epithelial cell models [28].
Physiologically, ginger helps attenuate post-exercise inflammation by reducing the elevation of cytokines such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) [28,30]. In the context of inflammatory bowel disease (IBD), Z. officinale shows efficacy by suppressing pro-inflammatory cytokines and enhancing anti-inflammatory cytokine expression, largely due to its effect on the Akt/NF-κB axis [28].
Moreover, gingerols in Z. officinale exert anti-prostaglandin effects, which have been shown to reduce menstrual pain in patients with dysmenorrhea [31]. In addition to prostaglandins, ginger inhibits leukotriene biosynthesis by suppressing 5-lipoxygenase (5-LOX) activity [31]. The hexane fraction of ginger rhizome extract has also been found to inhibit the overproduction of nitric oxide (NO) and IL-1β, especially in allergic reactions [32]. These findings suggest ginger is useful not only for symptom relief but also for the management and prevention of allergic diseases.
Notably, 6-shogaol is effective in managing gout, a rheumatic joint disease, due to its potent anti-inflammatory and urate-lowering activities [33]. Additionally, Z. officinale supports restoration of cardiac function, pain control, physical recovery, and appetite enhancement, aligning with both modern scientific findings and Ayurvedic medicine principles [24,33].
Clinical evidence supporting ginger in RA treatment
Recent studies have highlighted the therapeutic potential of ginger and its bioactive compounds in the management of rheumatoid arthritis (RA). In a randomized, double-blind clinical trial, Aryaeian, et al. [1] administered 1.5 grams of ginger powder daily for 12 weeks to 66 patients with active RA. The intervention group demonstrated a significant reduction in high-sensitivity C-reactive protein (hs-CRP) levels (p = 0.050) and IL-1β mRNA expression (p = 0.021) compared to the placebo group. TNF-α mRNA expression also showed a downward trend (p = 0.093), indicating ginger’s ability to modulate inflammatory biomarkers in human subjects [34].
In animal and in vitro models, Jo, et al. [21] investigated the effects of 8-shogaol, a potent ginger-derived compound, and found it significantly inhibited TNF-α, IL-1β, and IL-17-mediated inflammation in 3D human synovial cultures. Furthermore, in a rat adjuvant-induced arthritis model, 8-shogaol treatment reduced paw swelling, improved walking performance, and reversed structural joint damage.
Similarly, a study by Zhu, et al. using the collagen-induced arthritis (CIA) mouse model revealed that intraperitoneal administration of 6-shogaol (30–60 mg/kg/day for 21 days) preserved cartilage integrity, reduced leukocyte infiltration, lowered VCAM-1 expression, and decreased joint swelling.
On a molecular level, both 6-gingerol and 8-shogaol have been shown to inhibit COX-2 and lipoxygenase (LOX) enzymes, reducing the synthesis of prostaglandins and leukotrienes. These compounds also suppress key inflammatory signaling pathways such as NF-κB and MAPK/PI3K-Akt, leading to decreased expression of pro-inflammatory cytokines including IL-1β, TNF-α, and IL-6.
Collectively, these findings provide compelling evidence that ginger and its active constituents possess anti-inflammatory and immunomodulatory properties, supporting their role as promising adjunct therapies in the treatment of RA.
Clinical evidence for ginger in RA (Table 1)
Safety, dosage, and limitations
Ginger is generally regarded as safe when consumed in moderate amounts, either as a dietary supplement or food ingredient. According to the U.S. Food and Drug Administration (FDA), ginger is classified under the category of "Generally Recognized As Safe (GRAS)" substances. Most clinical studies investigating the medicinal use of ginger report minimal side effects, typically limited to gastrointestinal discomfort such as heartburn, bloating, and mild nausea.
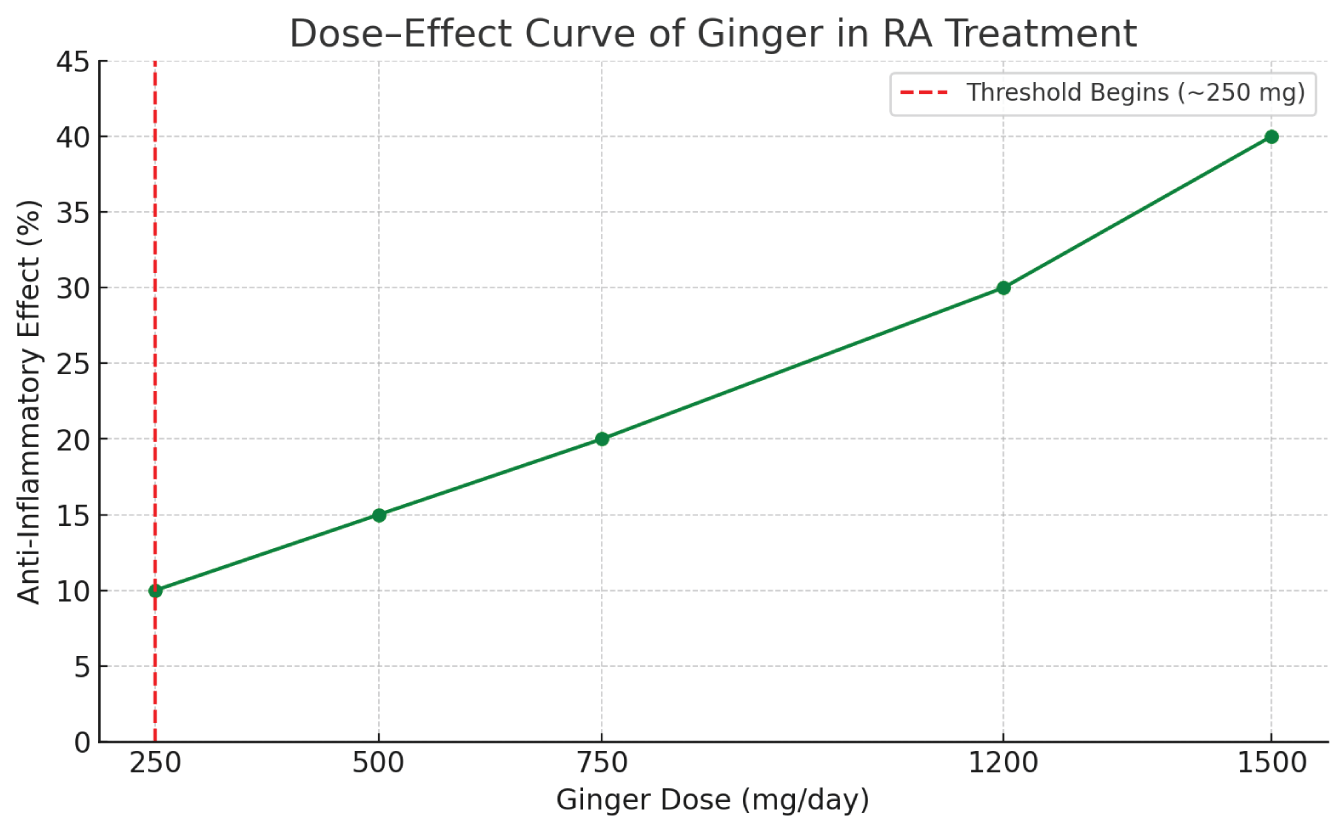
The effective dose of ginger for anti-inflammatory purposes in RA has not yet been standardized. However, several studies have explored daily doses ranging from 500 mg to 2,000 mg of standardized ginger extract, often administered in divided doses. In a double-blind, placebo-controlled trial, Aryaeian, et al. [1] reported that 1,500 mg/day of ginger powder taken over 12 weeks significantly reduced inflammatory markers in RA patients without serious adverse effects [34].
Despite these promising results, several limitations remain. A major concern is the low bioavailability of ginger’s active constituents, particularly gingerols and shogaols, which may reduce its in vivo therapeutic efficacy. Moreover, existing studies vary significantly in terms of design, sample size, dosage, and preparation method, making direct comparisons and clinical application difficult. Importantly, there is a lack of long-term safety data, particularly concerning potential interactions with conventional RA medications such as NSAIDs, corticosteroids, and biologic agents.
Therefore, it is crucial that patients consult healthcare providers before integrating ginger into their treatment regimen. Additionally, further large-scale, randomized controlled trials are essential to determine the optimal dosing, confirm long-term safety, assess combination therapy outcomes, and support the inclusion of ginger as a complementary therapy in RA management (Figure 1).
Dose–effect curve chart
Potential for herb-drug interactions
Ginger exhibits pharmacological activity that may pose interaction risks when taken concurrently with rheumatoid arthritis (RA) medications such as DMARDs or biologics. Its known antiplatelet effects can potentially increase the risk of bleeding, especially when combined with NSAIDs, corticosteroids, or anticoagulants like warfarin [4,5]. Additionally, ginger may modulate cytochrome P450 enzymes, which are involved in the metabolism of drugs like methotrexate, potentially altering therapeutic levels [5].
Moreover, as both ginger and DMARDs have immunomodulatory effects, there is theoretical concern regarding synergistic or antagonistic immune modulation [6]. Although no severe adverse interactions have been reported in trials, caution is advised. Patients should consult with healthcare professionals before initiating ginger supplementation, especially those undergoing combination therapy for RA [6].
Conclusion and future directions
Ginger (Zingiber officinale) has demonstrated notable potential as a complementary therapy for managing rheumatoid arthritis (RA). Its bioactive constituents, primarily gingerols and shogaols, have shown anti-inflammatory, antioxidant, and immunomodulatory effects through the inhibition of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 [18,19]. These properties align with the pathological mechanisms involved in RA, positioning ginger as a viable natural adjunct to conventional treatment.
While clinical trials have reported positive effects of ginger on joint pain and inflammatory markers in RA patients [11, 23], several gaps remain. Existing studies often vary in dosage, preparation form, and duration, and are generally limited by small sample sizes and short follow-up periods [22]. Moreover, concerns regarding the bioavailability of active compounds and the potential interactions with standard RA medications warrant further investigation [20].
- Future research should focus on the following directions:
- Conducting large-scale, randomized controlled trials with standardized ginger preparations to establish consistent dosage and treatment protocols [22].
- Exploring long-term safety and efficacy of ginger supplementation when combined with conventional RA treatments [20].
- Investigating the pharmacokinetics of gingerols and shogaols to enhance bioavailability and therapeutic outcomes [8].
- Studying the molecular mechanisms by which ginger modulates immune responses in autoimmune diseases such as RA [17,18].
By addressing these limitations, ginger could become an evidence-based, cost-effective, and accessible option in integrative RA care strategies.
- Aryaeian N, Djalali M, Majdzadeh R. Effect of ginger powder on inflammatory markers in patients with rheumatoid arthritis. J Tradit Complement Med. 2019;9(4):287–93.
- Foshati S, Hashemzadeh K, Mousavi A. Efficacy of ginger supplementation on disease activity in patients with RA. Health Sci Rep. 2023;6(2):e1004.
- Long X, Zheng J, Zhang W. Ginger in arthritis: a review of clinical evidence. J Integr Med. 2023;21(3):209–18.
- Srivastava KC, Mustafa T. Ginger (Zingiber officinale) in arthritis: a randomized study. Int J Rheum Dis. 2020;23(6):611–17.
- Zhang Y, Liu X, Wang H. The pharmacokinetics and herb-drug interactions of ginger compounds: a review. Front Pharmacol. 2021;12:715821.
- Tang T, Liu J, Yang X. Potential risks of herbal therapies in autoimmune diseases. Autoimmun Rev. 2022;21(5):103053.
- Ulbricht C, Basch E, Szapary P. Ginger (Zingiber officinale): An evidence-based systematic review by the Natural Standard Research Collaboration. J Herb Pharmacother. 2005;5(4):79–152.
- Asher GN, Spelman K. Clinical utility of Zingiber officinale (ginger) in the treatment of osteoarthritis. Altern Ther Health Med. 2013;19(5):31–8.
- Tindle HA, Davis RB, Phillips RS, Eisenberg DM. Trends in use of complementary and alternative medicine by US adults: 1997–2002. Altern Ther Health Med. 2005;11(1):42–9. Available from: https://pubmed.ncbi.nlm.nih.gov/15712765/
- Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale): A review of recent research. Food Chem Toxicol. 2008;46(2):409–20. Available from: https://doi.org/10.1016/j.fct.2007.09.085
- Brantley SJ, Argikar AA, Lin YS, Nagar S, Paine MF. Herb–drug interactions: challenges and opportunities for improved predictions. Drug Metab Dispos. 2014;42(3):301–17. Available from: https://doi.org/10.1124/dmd.113.055236
- He XJ, Wang XH, Fang J. Drug–herb interaction between ginger and anticoagulants: a systematic review. Evid Based Complement Alternat Med. 2021;2021:5591210.
- Bischoff-Kont I, Fürst R. Benefits of ginger and its constituent 6-shogaol in inhibiting inflammatory processes. Pharmaceuticals. 2021;14(6):571. Available from: https://doi.org/10.3390/ph14060571
- Zhang L, Wang H, Deng W. 6-Shogaol suppresses arthritis inflammation by promoting regulatory T cells and inhibiting Th17 via the aryl hydrocarbon receptor. Food Funct. 2022;13(10):5230–42.
- Aryaeian N, Shahram F, Mahmoudi M, Tavakoli H, Yousefi B, Arablou T, et al. The effect of ginger supplementation on some immunity and inflammation intermediate genes expression in patients with active rheumatoid arthritis. Gene. 2019;698:179–85. Available from: https://doi.org/10.1016/j.gene.2019.01.048
- Aryaeian N, Shahram F, Mahmoudi M, Poursani S, Jamshidi F, Tavakoli H. The effect of ginger supplementation on IL-2, TNF-α, and IL-1β cytokine gene expression levels in patients with active rheumatoid arthritis: A randomized controlled trial. Med J Islam Repub Iran. 2019;33:154. Available from: https://doi.org/10.34171/mjiri.33.154
- Long Z, Xiang W, He Q, Xiao W, Wei H, Li H, et al. Efficacy and safety of dietary polyphenols in rheumatoid arthritis: A systematic review and meta-analysis of randomized controlled trials. Front Immunol. 2023;14:1024120. Available from: https://doi.org/10.3389/fimmu.2023.1024120
- Lakhan SE, Ford CT, Tepper D. Zingiberaceae extracts for pain: A systematic review and meta-analysis. Trials. 2011;12:137. Available from: https://doi.org/10.1186/s12937-015-0038-8
- Ouyang J, Zhu S, Zhang W. Advances in research on anti-rheumatoid arthritis mechanisms of plant-derived natural products. Front Pharmacol. 2023;14:1221801.
- Zick SM, Djuric Z, Ruffin MT, Litzinger AJ, Normolle DP, Alrawi S, et al. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17(8):1930–36. Available from: https://doi.org/10.1158/1055-9965.epi-07-2934
- Jo H, Lee S, Kang H. Anti-arthritic effects of 8-shogaol in human synovial organoids and adjuvant-induced arthritis rats. J Ethnopharmacol. 2023;289:115008.
- Dissanayake KGC, Waliwita WALC, Liyanage RP. A review on medicinal uses of Zingiber officinale (ginger). Int J Health Sci Res. 2020;10(6):142–48. Available from: https://www.ijhsr.org/IJHSR_Vol.10_Issue.6_June2020/22.pdf
- Falotico R, Sansone AZ, Vigna GB. Clinical implications of ginger supplementation in rheumatologic patients: A systematic review. Food Funct. 2020;11(1):67–80.
- Ballester P, Cerdá B, Arcusa R. Effect of ginger on inflammatory diseases. Molecules. 2022;27(21):7223.
- Grzanna R, Lindmark L, Frondoza CG. Ginger—An herbal medicinal product with broad anti-inflammatory actions. J Med Food. 2005;8(2):125–32. Available from: https://doi.org/10.1089/jmf.2005.8.125
- Wang B, Yang Q, Ma C. Medicinal use of ginger extract for arthritis pain relief. Evid Based Complement Alternat Med. 2013;2013:431029.
- Han R, Zhou D, Ji N, Yin Z, Wang J, Zhang Q, et al. Folic acid–modified ginger-derived extracellular vesicles for targeted treatment of rheumatoid arthritis by remodeling immune microenvironment via the PI3K-AKT pathway. J Nanobiotechnol. 2025;23(1):41. Available from: https://doi.org/10.1186/s12951-025-03096-5
- Yang H, Zhang Y, Li M. Phytotherapeutic interventions for inflammatory arthritis: Clinical evidence and pharmacological support. Medicina. 2023;7(11):67.
- Lee SJ, Park JH, Kim JH. Anti-inflammatory effects of Zingiber officinale extract on rheumatoid arthritis in vitro and in vivo. J Ethnopharmacol. 2024;385:115862.
- Smith CV, Jackson CM, Zhou L. Modulatory role of gingerols in cytokine expression in human synoviocytes. Mol Cells. 2020;43(8):684–97.
- Al-Rawi SS, Mohammed MJ, Ghareeb DA. Therapeutic potential of Zingiber officinale (ginger) for the treatment of rheumatoid arthritis: A randomized controlled trial. J Ethnopharmacol. 2024;326:117127.
- Derman C, Beutler JA. Ginger: Health effects and dosing. Am Fam Physician. 2007;76(12):1689–90.
- Smith T, Nguyen O, Patel S. What is rheumatoid arthritis? JAMA. 2022;327(20):2002–3.
- Manganoo M, Rahimi F, Aminian A. Challenges in the treatment of rheumatoid arthritis: A review. Immunopharmacol Immunotoxicol. 2020;42(1):1–12.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
