Open Journal of Orthopedics and Rheumatology
Amniotic derived exosomes in the treatment of degenerative bone- and cartilage disease: A case series
Torbjörn Ogéus*
Cite this as
Ogéus T (2024) Amniotic derived exosomes in the treatment of degenerative bone- and cartilage disease: A case series. Open J Orthop Rheumatol 9(1): 001-006. DOI: 10.17352/ojor.000049Copyright License
© 2024 Ogéus T. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction: Exosomes, nanosized vesicles derived from various cellular sources, hold immense potential in modulating physiological processes and promoting tissue repair. Exosomes derived from amniotic fluid show significant promise in regenerative medicine and tissue repair. To our knowledge, there are no reported cases of reduction of chronic pain due to bone- and cartilage degeneration reported after exosome treatment.
Case presentation: Three male patients, all with chronic pain and reduced physical function due to bone- or cartilage degeneration all showed a remarkable reduction in pain and increase in function after one single injection of amniotic-derived exosomes.
Conclusion: These cases raise the hypothesis that amniotic-derived exosomes may be an effective intervention in the treatment of degenerative bone- and cartilage diseases. Especially in combination with other regenerative medicine interventions. Further research is required to test this theory.
Introduction
Regenerative medicine, aimed at harnessing the body's innate ability to heal and repair tissues, has witnessed a surge in interest and research efforts in recent years. Central to this field is the exploration of extracellular vesicles, particularly exosomes, as promising mediators of intercellular communication and tissue regeneration [1]. Exosomes, nanosized vesicles derived from various cellular sources, hold immense potential in modulating physiological processes and promoting tissue repair.
Exosomes derived from amniotic fluid, have emerged as pivotal mediators in intercellular communication, holding significant promise in regenerative medicine and tissue repair [2]. These lipid bilayer-enclosed vesicles carry a cargo of proteins, lipids, nucleic acids, and other bioactive molecules reflective of their cell of origin. Their role in modulating physiological processes has garnered considerable attention in recent years.
Exosomes derived from amniotic fluid are implicated in various cellular functions, including cell proliferation, differentiation, and immunomodulation [3]. They participate in cross-talk between different cell populations within the microenvironment, influencing cellular responses crucial for tissue homeostasis and regeneration. Studies have demonstrated that exosomes derived from amniotic fluid possess potent pro-regenerative properties, promoting tissue repair and modulating inflammatory responses [4].
Moreover, exosomes offer unique advantages over their parent cells in therapeutic applications, including enhanced stability, reduced immunogenicity, and the ability to traverse biological barriers [5]. Harnessing the regenerative potential of exosomes derived from amniotic fluid holds promise for developing novel therapeutic strategies for addressing various pathological conditions.
Several mechanisms underlie the therapeutic effects of exosomes derived from amniotic fluid, including the transfer of bioactive molecules, modulation of signaling pathways, and regulation of immune responses [6]. Understanding the complex interplay between exosomes and recipient cells is crucial for optimizing their therapeutic efficacy and translating them into clinical practice.
This case series highlights effects in joint healing previously unpublished where exosomes derived from amniotic fluid promote tissue regeneration in bone and cartilage tissues.
Case presentations
Case 1
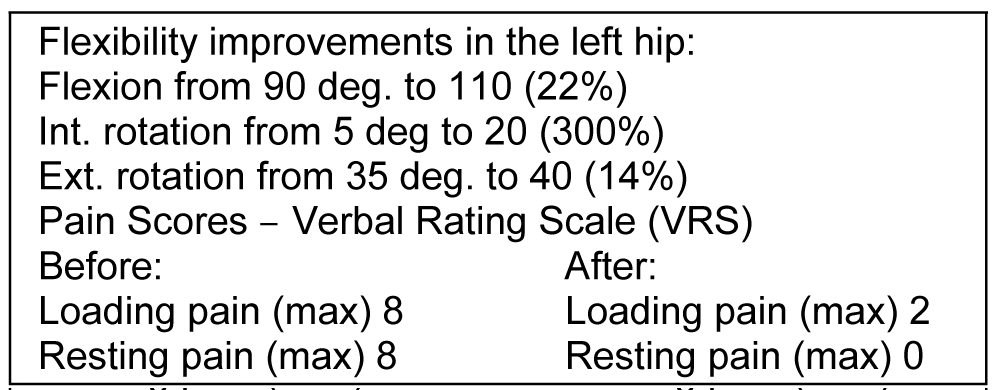
Our first patient was a 32-year-old man who presented at the clinic with chronic hip pain and radiographically verified osteoarthritis (OA) of the left hip joint. He had a history of Perthes disease and had undergone 5 surgical interventions over the years he presented symptoms typical for hip osteoarthritis such as restricted movement in flexion, internal- as well as external rotation, and pain both at resting and while loading and weight-bearing. He is a professional basketball player, but at the time he came to the clinic, he was unable to play or exercise due to the pain and restrictions. He reported that his pain at exercising was 8 on the Verbal Rating Scale (VRS) and resting pain after professional basketball games in the last year was between 6-8 on the VRS scale. He was considering ending his professional career due to the pain.
Case 2
Our second patient was a 33-year-old man, he was a former professional ice hockey player but had to end his career 7 years earlier due to multiple lower back injuries with 4 separate disc hernias in the lower back and severely reduced disc space as a result. Since the injuries, he experienced constant chronic pain in the lower back every day with periods of sharper and stronger pain. He reported that his pain level was constantly between 3 and 7 on the VRS scale.
Case 3
Our third patient was a 54-year-old man with multiple injuries and chronic pain after a motocross injury 35 years earlier where he fractured a vertebra in the thoracic spine (Th 4), his left shoulder had partial tears of the rotator cuff, his left hip had signs of earlier fracture and the left knee had severe medial osteoarthritis. Upon presentation he reported that he had chronic pain in his thoracic spine since the accident when he was 19, the spinal pain was always present while moving or exercising and it made many movements and strenuous activities difficult. Upon presentation at the clinic, he reported that his knee made him wake up between 5 and 10 times every night and if he walked approximately 3km he had swelling and pain for about a week after.
All three cases had different injuries and anamnesis and therefore received different treatments for their conditions, however, they all had chronic pain due to bone- or cartilage degeneration and they all received a single injection of amniotic fluid-derived exosomes and experienced similar intriguing responses in terms of long-term symptom relief of chronic pain.
Materials and methods
Various types of autologous platelet concentration have been proposed to be used to treat osteoarthritis and cartilage defects. Some studies have indicated a positive effect on pain, function, and stiffness symptoms however, the effect of injected platelets in the injured joint might require multiple injections and no standard protocols exist [7]. One way of extending the effect of the injected platelets is to heat a liquid platelet-poor plasma (PPP) layer, the resorption properties of heated albumin (albumin gel) can be extended from 2 weeks to greater than 4 months (ALB-PRF) [8].
The use of autologous mesenchymal stem cells harvested from the Stromal Vascular Fraction (SVF) has been used increasingly in the treatment of osteoarthritis with promising results in cartilage repair. The combination of autologous blood products such as PRF intra-articular has shown beneficial effects for OA treatment [9].
Exosomes derived from stem cells can recapitulate the potential of their parent cells and have therefore been proposed as a substitute for cell therapy to achieve a cell-free therapy option [10,11]. Different routes of administration have been evaluated and the most common route in preclinical studies was the intravenous (IV) injection [12].
Preparation of PRF, ALB-PRF, SVF and exosomes
40ml blood was collected from the patients before each of the PRF injections. Four 10ml Plastic, round-bottomed vacuum tubes (Liquid PRF tubes) were used to collect the blood, after collection the tubes were spun on a horizontal swing-out bucket rotors centrifuge system. Two PRF protocols were utilized in the treatment in this case series including a Concentrated-PRF (C-PRF) protocol of 2000xg for 8 min and a Heat-Coagulated Albumin Gel-PRF (ALB-PRF) protocol of 2000xg for 8 min followed by a heating and cooling down process before injection was performed. The two protocols were utilized following international guidelines for PRF utilization published by Miron, et al. in 2019 [13].
The PRF injections consisted of concentrated Platelet Rich Fibrin (C-PRF) [14] injection of 4ml, centrifuged at 2000×g for 8 min. While ALB-PRF injections given were 5ml ALB-PRF, 2000xg for 8 min on a horizontal centrifuge, the albumin layer was heated according to the ALB-PRF protocol; 75 degrees for 10 min [15]. In the last step, the heat-coagulated albumin gel was cooled down to room temperature and mixed with the remaining C-PRF to create ALB-PRF. The centrifuge utilized in all PRF treatments was the Bio-PRF horizontal centrifuge (Bio-PRF, USA).
The SVF preparation followed the MyStem™ system protocol (MyStem evo Bi-Medica, Treviolo, Italy). 80 ml of adipose tissue from the abdomen was harvested through liposuction and then added to the MyStem system, after filtration and wash cycles, the residual fluid and oil were removed. 10 ml of the SVF suspension was extracted and prepared for injection.
The Exosomes used were 4 trillion amniotic-derived exosomes in a 1,5ml extracellular matrix (The Center for Regenerative Medicine Laboratories, Miami, USA), in case 1 and 3 half of the matrix was diluted with 5 ml Saline for intravenous injection, while the other half was combined with PRF for intra-articular injection. In case 2 all the extracellular matrix was diluted in Saline for intravenous injection.
Administration of PRF, ALB-PRF and SVF
The patients in both case 1 and case 3 underwent a series of intra-articular C-PRF, ALB-PRF injections, and one intra-articular SVF injection in the osteoarthritic joints. In the first week one intra-articular C-PRF injection two days before the SVF treatment followed by two intra-articular C-PRF injections in the following week spaced two days apart, finishing with one intra-articular ALB-PRF injection. The treated joins were instructed to avoid weight bearing for 5 weeks using crutches. All intra-articular joint injections were performed with ultrasound guidance to ensure needle placement inside the joint capsule.
Administration of the exosomes
In cases 1 and 3 the exosomes were divided in half, where half of the injection was intra-articular in the osteoarthritic joint combined with a C-PRF injection, and half of the exosomes were diluted in saline for a systemic injection intravenously (IV) using the IV-Push technique [16]. In case 2, all 4 trillion exosomes were diluted in saline and injected intravenously with the IV-Push technique, no allergic or adverse reactions were observed after any of the injections.
Results
Case 1
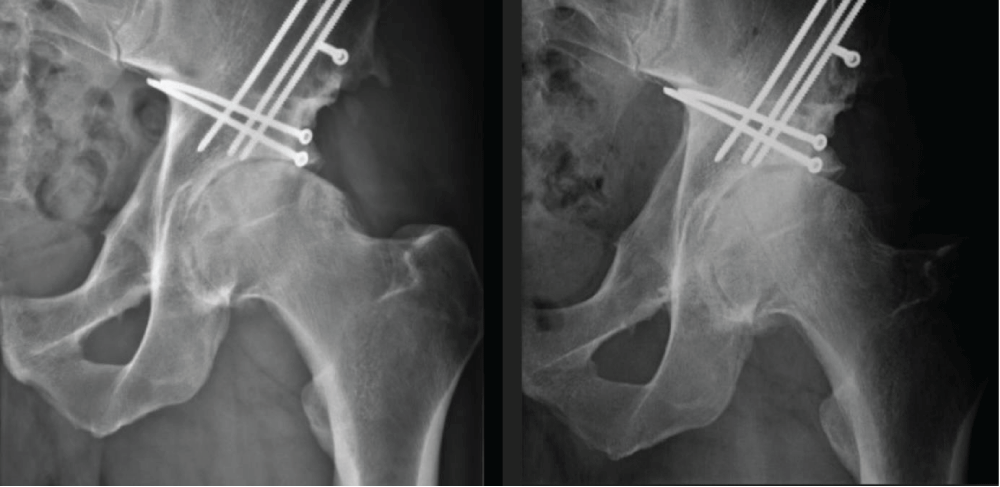
6 months after the PRF, SVF, and exosome treatment of the hip. His flexibility and pain levels had improved significantly (Figure 1), and he was able to return to professional basketball where he is still currently active one year later. Radiographic pictures were taken to compare the status before- and after the treatment an increase of approximately 1.5-2 mm in the joint space was seen cranially (Figure 2).
Case 2
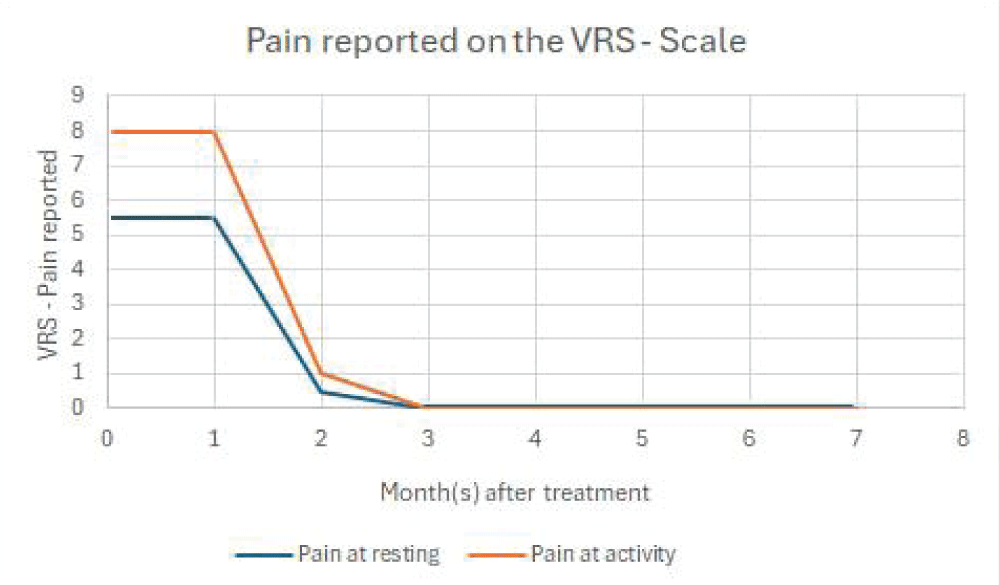
3 weeks after the intravenous exosome treatment, the patient reported that “all former back pain was gone”. 6 months later he still reported that the back was completely pain-free. Situations and activities that usually caused pain for the last 7 years are now pain-free, he reported that he had been able to exercise and train in the gym on a level that was impossible since his injuries (Figure 3).
Case 3
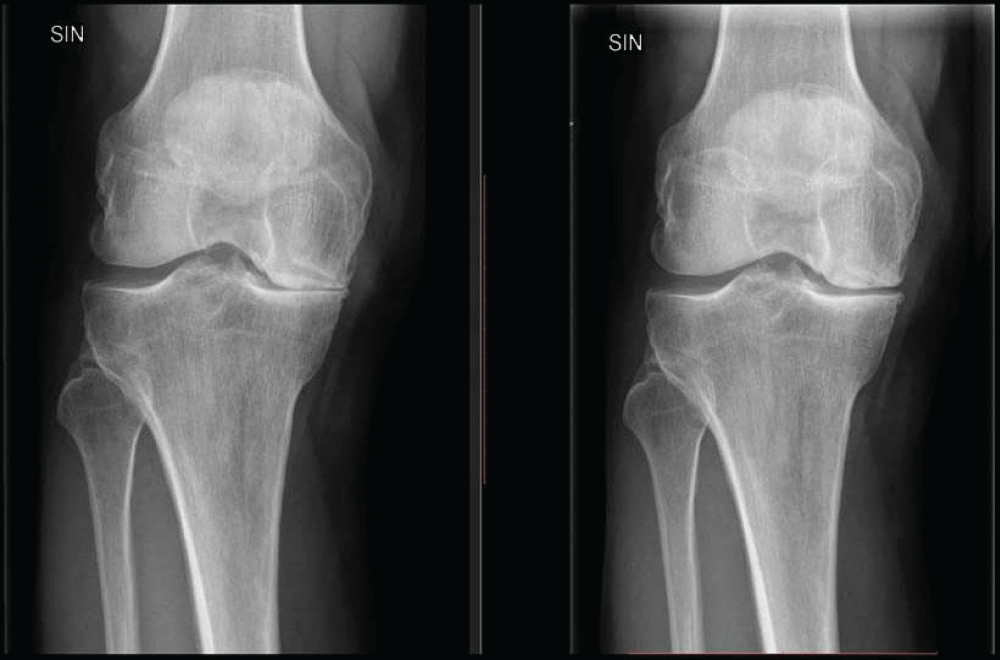
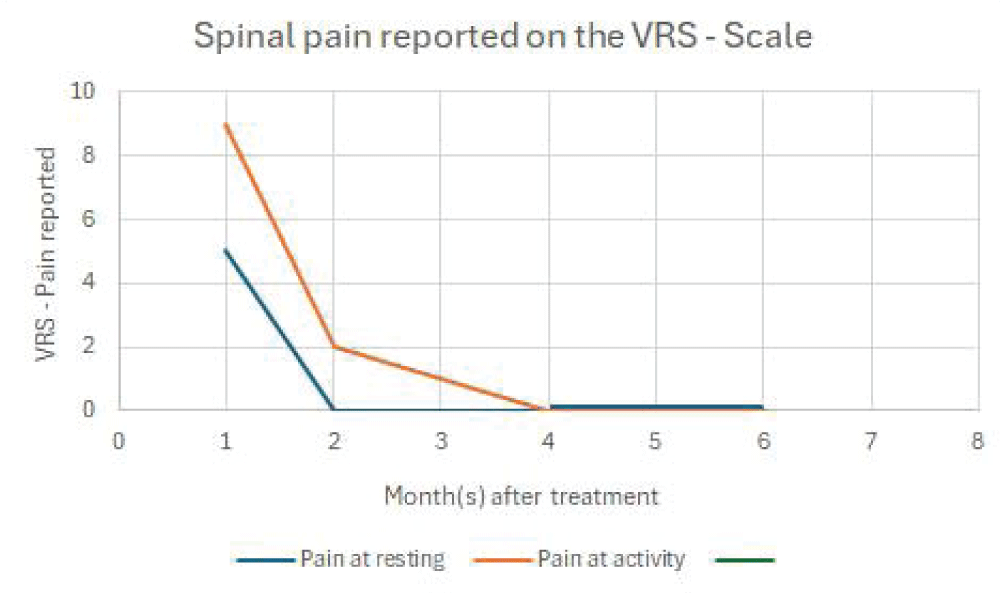
1 month after the PRF, SVF, and exosome treatment the patient reported that he “hasn’t had any spinal pain for weeks” This was the first time in 35 years that he didn’t experience pain in the area where his vertebra Th4 was fractured. 3 months and 6 months later he reported the same thing. He was able to exercise and move in ways that had been impossible because of the back pain for 35 years. His knee pain had also improved significantly. Before the treatment, he reported that if he walked 3km he would experience swelling and pain for weeks after. 6 months after the treatment series he reported that he was able to walk 10km or more without any swelling or pain at all. He also reported that he hadn´t been woken up by pain in the knee at all for many months (Figure 5). A radiographic examination of the left knee was performed to evaluate the difference before- and after the treatment series. The former joint space narrowing on the medial side of the knee had now increased by approximately 2 mm (Figure 4).
Discussion
The cases presented herein describe a successful novel use of a combination of PRF, ALB-PRF, SVF, and amniotic-derived exosomes for osteoarthritis and a treatment with amniotic-derived exosomes against chronic spinal pain and degeneration, thus providing us insight into an alternative intervention for patients where the standard conservative treatment has failed or as an option to surgical intervention.
SVF treatment alone or in combination with platelet injections has been studied and has seen a growing level of evidence for its use, especially for symptom alleviation in the short term for knee osteoarthritis [17]. In this case series, two individual cases of osteoarthritis showed an increase in joint space on radiographic pictures, and the reported pain levels drastically lowered within a month after treatment.
The use of exosomes has been proposed in both bone and tendon healing for their extraordinary regenerative potential, however, clinical data is still scarce and most studies focus on preclinical data [18]. Numerous studies have also focused on the regenerative potential exosomes have on cartilage [19,20]. In the cases in this study, amniotic-derived exosomes were used as an addition to SVF and PRF treatments with extraordinary results. However, the positive effects on chronic spinal pain in degenerative conditions in two of the reported cases were intriguing and showed a somewhat unexpected but highly positive result.
The range of potential uses of the exosomes raises interesting regulatory questions and challenges. Although derived from cells and containing nucleic acids, exosomes will not be considered Advanced Therapy Medicinal Products (ATMPs) in Europe, unless they are used to deliver gene therapy or as a drug delivery vehicle [21].
Spinal injuries and degeneration of discs and vertebras have been proven to be difficult to effectively treat and hard to successfully cure. The risk of interventions such as injections or surgery on spinal injuries is higher than with peripheral joints and requires high specialization
[22]. Mesenchymal-derived exosomes have been utilized as a stand-alone treatment of degenerative disc disease with positive results after administration of a single dose systemically, however, data is missing for multiple infusions [23]. In a recent study, the effects of different stem cell and exosome-based therapies on intervertebral disc disease were compared, and concluded that the initial effects from studies were promising however the dosage for a particular disease treatment is not determined, and individual reactions might be unpredictable [24].
The intravenous IV-Push injection of exosomes may be one minimally invasive way to treat spinal conditions in certain cases. The common denominator in the two successfully treated cases from this study was that they were old spinal injuries where chronic pain developed over time. This case series focused on the first 6 to 12 months after the treatments, a longer follow-up time in future studies is desired and needed to evaluate and optimize the protocol. A larger patient group is also needed to further test the theories in full. However, the conditions in all three cases were chronic, treatment-resistant conditions, with little to no chances of spontaneous healing while presenting to the clinic and the positive results were significant.
Exosome therapy is still in its infancy and the appropriate dosage and protocols are still to be determined. Exosomes are becoming a novel and promising cell-free therapeutic tool in multiple ongoing clinical studies and have demonstrated safety and potential efficacy in a handful of reported clinical studies. On the other hand, exosomes face challenges regarding their clinical applications, such as product heterogeneity, uncertain clearance from the body, and long-term preservation stability. there is also an urgent need to standardize the therapeutic doses and route of administration [25]. Long-term effects are still unknown and especially if repeated or prolonged administration, remain poorly understood. Longitudinal studies tracking the fate of exosomes in vivo and monitoring for late-onset adverse events are necessary to assess the long-term safety profile of exosome therapy. Standardization and quality control of exosome isolation, purification, and characterization are critical. Variability in isolation techniques, cell sources, and purification methods can affect the composition and efficacy of exosome preparations, potentially leading to inconsistent therapeutic outcomes or safety issues [26].
The cases in this study show a remarkable short-term effect on back pain and osteoarthritic pain, however, the long-term effects are still unsure due to the time limitation of the available data, future studies where the long-term effects and potential adverse effects might occur are warranted before a treatment protocol can be proposed. Also, the use of more standardized and validated measurement tools in future studies, such as Western Ontario and McMaster University Arthritis Index (WOMAC) [27] might be used to compare the results to other modalities and interventions previously published.
One of the main advantages of exosome therapy is the minimally invasive approach, where the risks of a conventional intervention such as surgery can be avoided and at a relatively low cost in comparison, however, the outcome of surgeries with years of studies behind it is difficult to match with the uncertainty the exosome therapy effects present today.
Conclusion
In conclusion, based on the experience of this case series and a review of the current specialist literature, we advise that amniotic-derived exosomes may be an effective intervention in the treatment of degenerative bone- and cartilage diseases. Especially in combination with other regenerative medicine interventions. Further research is required to test this theory.
Declaration
Ethics approval: According to the Ethics Commission of Stockholm, Sweden, case reports with biological medicinal products do not require ethical approval for publication. This applies to the present study.
The study was conducted following the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent for publication: Written informed consent was obtained from the patients for the publication of these case reports and any accompanying images. Copies of the written consent forms are available for review by the Editor-in-Chief of this journal.
The author was the main and only contributor to the manuscript.
Authors’ contributions
TO was the patient’s primary caregiver regarding the specific injuries. All texts, design, literature review, and drafting of this case report were done by TO, responsible for the submitted manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files]. Radiographic and ultrasound images are stored at the clinic and are available for review by the Editor-in-Chief of this journal.
- Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int J Mol Sci. 2017 Aug 25;18(9):1852. doi: 10.3390/ijms18091852. PMID: 28841158; PMCID: PMC5618501.
- EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013 May;12(5):347-57. doi: 10.1038/nrd3978. Epub 2013 Apr 15. PMID: 23584393.
- Witwer KW, Van Balkom BWM, Bruno S, Choo A, Dominici M, Gimona M, Hill AF, De Kleijn D, Koh M, Lai RC, Mitsialis SA, Ortiz LA, Rohde E, Asada T, Toh WS, Weiss DJ, Zheng L, Giebel B, Lim SK. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles. 2019 Apr 29;8(1):1609206. doi: 10.1080/20013078.2019.1609206. PMID: 31069028; PMCID: PMC6493293.
- Zavatti M, Beretti F, Casciaro F, Bertucci E, Maraldi T. Comparison of the therapeutic effect of amniotic fluid stem cells and their exosomes on monoiodoacetate-induced animal model of osteoarthritis. Biofactors. 2020 Jan;46(1):106-117. doi: 10.1002/biof.1576. Epub 2019 Oct 18. PMID: 31625201.
- Murphy SV, Shiyun SC, Tan JL, Chan S, Jen H, Wang S, Choolani M. Amnion epithelial cell-derived exosomes restrict lung injury and enhance endogenous lung repair. Stem Cells Translational Medicine. 2019; 8(3):334-344.
- Ilancheran S, Moodley Y, Manuelpillai U. Human fetal membranes: a source of stem cells for tissue regeneration and repair? Placenta. 2009 Jan;30(1):2-10. doi: 10.1016/j.placenta.2008.09.009. Epub 2008 Nov 7. PMID: 18995896.
- Costa LAV, Lenza M, Irrgang JJ, Fu FH, Ferretti M. How Does Platelet-Rich Plasma Compare Clinically to Other Therapies in the Treatment of Knee Osteoarthritis? A Systematic Review and Meta-analysis. Am J Sports Med. 2023 Mar;51(4):1074-1086. doi: 10.1177/03635465211062243. Epub 2022 Mar 22. PMID: 35316112.
- Fujioka-Kobayashi M, Schaller B, Mourão CFAB, Zhang Y, Sculean A, Miron RJ. Biological characterization of an injectable platelet-rich fibrin mixture consisting of autologous albumin gel and liquid platelet-rich fibrin (Alb-PRF). Platelets. 2021 Jan 2;32(1):74-81. doi: 10.1080/09537104.2020.1717455. Epub 2020 Jan 20. PMID: 31959025.
- Mehranfar S, Abdi Rad I, Mostafav E, Akbarzadeh A. The use of stromal vascular fraction (SVF), platelet-rich plasma (PRP) and stem cells in the treatment of osteoarthritis: an overview of clinical trials. Artif Cells Nanomed Biotechnol. 2019 Dec;47(1):882-890. doi: 10.1080/21691401.2019.1576710. PMID: 30887856.
- Bagno L, Hatzistergos KE, Balkan W, Hare JM. Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Mol Ther. 2018 Jul 5;26(7):1610-1623. doi: 10.1016/j.ymthe.2018.05.009. Epub 2018 May 25. PMID: 29807782; PMCID: PMC6037203.
- Lou G, Chen Z, Zheng M, Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. 2017 Jun 16;49(6):e346. doi: 10.1038/emm.2017.63. PMID: 28620221; PMCID: PMC5519012.
- Hassanzadeh A, Rahman HS, Markov A, Endjun JJ, Zekiy AO, Chartrand MS, Beheshtkhoo N, Kouhbanani MAJ, Marofi F, Nikoo M, Jarahian M. Mesenchymal stem/stromal cell-derived exosomes in regenerative medicine and cancer; overview of development, challenges, and opportunities. Stem Cell Res Ther. 2021 May 21;12(1):297. doi: 10.1186/s13287-021-02378-7. PMID: 34020704; PMCID: PMC8138094.
- Miron RJ, Pinto NR, Quirynen M, Ghanaati S. Standardization of relative centrifugal forces in studies related to platelet-rich fibrin. J Periodontol. 2019 Aug;90(8):817-820. doi: 10.1002/JPER.18-0553. Epub 2019 Mar 1. PMID: 30730050.
- Fujioka-Kobayashi M, Katagiri H, Kono M, Schaller B, Zhang Y, Sculean A, Miron RJ. Improved growth factor delivery and cellular activity using concentrated platelet-rich fibrin (C-PRF) when compared with traditional injectable (i-PRF) protocols. Clin Oral Investig. 2020 Dec;24(12):4373-4383. doi: 10.1007/s00784-020-03303-7. Epub 2020 May 7. PMID: 32382929.
- Miron RJ, Pikos MA, Estrin NE, Kobayashi-Fujioka M, Espinoza AR, Basma H, Zhang Y. Extended platelet-rich fibrin. Periodontol 2000. 2023 Nov 20. doi: 10.1111/prd.12537. Epub ahead of print. PMID: 37986559.
- Burger M, Degnan D. Comparative Safety, Efficiency, and Nursing Preference Among 3 Methods for Intravenous Push Medication Preparation: A Randomized Crossover Simulation Study. J Patient Saf. 2019 Sep;15(3):238-245. doi: 10.1097/PTS.0000000000000269. PMID: 27128107; PMCID: PMC6727910.
- Di Matteo B, Vandenbulcke F, Vitale ND, Iacono F, Ashmore K, Marcacci M, Kon E. Minimally Manipulated Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Systematic Review of Clinical Evidence. Stem Cells Int. 2019 Aug 14;2019:1735242. doi: 10.1155/2019/1735242. PMID: 31485234; PMCID: PMC6710724.
- Zou M, Wang J, Shao Z. Therapeutic Potential of Exosomes in Tendon and Tendon-Bone Healing: A Systematic Review of Preclinical Studies. J Funct Biomater. 2023 May 28;14(6):299. doi: 10.3390/jfb14060299. PMID: 37367263; PMCID: PMC10299056.
- Zhang P, Dong B, Yuan P, Li X. Human umbilical cord mesenchymal stem cells promoting knee joint chondrogenesis for the treatment of knee osteoarthritis: a systematic review. J Orthop Surg Res. 2023 Aug 29;18(1):639. doi: 10.1186/s13018-023-04131-7. PMID: 37644595; PMCID: PMC10466768.
- D'Arrigo D, Roffi A, Cucchiarini M, Moretti M, Candrian C, Filardo G. Secretome and Extracellular Vesicles as New Biological Therapies for Knee Osteoarthritis: A Systematic Review. J Clin Med. 2019 Nov 4;8(11):1867. doi: 10.3390/jcm8111867. PMID: 31689923; PMCID: PMC6912212.
- Stapleton S. Exosomes as therapeutics and drug delivery vehicles: global regulatory perspectives. Cell & Gene Therapy Insights 2020; 6(11):1561-1569.
- Corp N, Mansell G, Stynes S, Wynne-Jones G, Morsø L, Hill JC, van der Windt DA. Evidence-based treatment recommendations for neck and low back pain across Europe: A systematic review of guidelines. Eur J Pain. 2021 Feb;25(2):275-295. doi: 10.1002/ejp.1679. Epub 2020 Nov 12. PMID: 33064878; PMCID: PMC7839780.
- Bhujel B, Shin HE, Choi DJ, Han I. Mesenchymal Stem Cell-Derived Exosomes and Intervertebral Disc Regeneration: Review. Int J Mol Sci. 2022 Jun 30;23(13):7306. doi: 10.3390/ijms23137306. PMID: 35806304; PMCID: PMC9267028.
- Xia Y, Yang R, Hou Y, Wang H, Li Y, Zhu J, Fu C. Application of mesenchymal stem cell-derived exosomes from different sources in intervertebral disc degeneration. Front Bioeng Biotechnol. 2022 Oct 7;10:1019437. doi: 10.3389/fbioe.2022.1019437. PMID: 36277386; PMCID: PMC9585200.
- Lotfy A, AboQuella NM, Wang H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res Ther. 2023 Apr 7;14(1):66. doi: 10.1186/s13287-023-03287-7. PMID: 37024925; PMCID: PMC10079493.
- Wiklander OPB, Brennan MÁ, Lötvall J, Breakefield XO, El Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med. 2019 May 15;11(492):eaav8521. doi: 10.1126/scitranslmed.aav8521. PMID: 31092696; PMCID: PMC7104415.
- Woolacott NF, Corbett MS, Rice SJ. The use and reporting of WOMAC in the assessment of the benefit of physical therapies for the pain of osteoarthritis of the knee: findings from a systematic review of clinical trials. Rheumatology (Oxford). 2012 Aug;51(8):1440-6. doi: 10.1093/rheumatology/kes043. Epub 2012 Mar 30. PMID: 22467082.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
