Archives of Organ Transplantation
Recurrence of Immunotactoid Glomerulopathy with Monoclonal IgG3κ Deposits after Kidney Transplant
Katsuyuki Miki1, Maki Sumida2*, Kazuhiro Iwadoh1, Kazuho Honda2, Toru Murakami2, Ichiro Koyama2, Ichiro Nakajima3 and Shohei Fuchinoue3
2Department of Nephrology and Endocrinology, The University of Tokyo, Tokyo, Japan
3Department of Anatomy, Showa University School of Medicine, Tokyo Japan
Cite this as
Miki K, Sumida M, Iwadoh K, Honda K, Murakami T, et al. (2017) Recurrence of Immunotactoid Glomerulopathy with Monoclonal IgG3κ Deposits after Kidney Transplant. Arch Organ Transplant 2(1): 015-018. DOI: 10.17352/aot.000006We report a case of rapid recurrence of immunotactoid glomerulopathy (ITG) with monoclonal IgG3κ deposits in a transplanted renal graft. A 55-year-old hemodialysis male patient due to ITG underwent an ABO-incompatible living-donor kidney transplantation. Proteinuria (3.11 g/day) and increased serum creatinine (2.52 mg/dL) were detected on postoperative day (POD) 4 due to acute antibody-mediated rejection (aAMR). Even after treatment for aAMR, proteinuria increased again to 4.5 g/day because of a recurrent ITG with IgG3κ subclass deposits. He returned to maintenance hemodialysis 9 months after transplantation.
This case underlines the importance of preoperative monoclonal paraproteinuria test to predict an ITG recurrence in the renal graft.
Introduction
Immunotactoid glomerulopathy (ITG), first reported by Korbet et al. in 1985 as a glomerular deposition disease in which immunoglobulin-derived protein filaments deposit in the glomeruli and cause a range of nephritis [1], is a rare renal disease found in fewer than 1% of all kidney biopsies [2,3]. It exhibits pathological manifestations ranging from mesangial proliferative glomerulonephritis to membranoproliferative glomerulonephritis (MPGN). Immunostaining is frequently positive for immunoglobulin G (IgG) κ or λ light chains and complement C3. Different from amyloidosis, Congo red staining is negative in ITG [4]. Although some patients reportedly respond to steroid therapy [5], the treatment is ineffective in up to half of all cases. These patients develop end-stage renal failure within a few years after onset.
Few reports of kidney transplantation have been made for patients with ITG. Although ITG has a high rate of recurrence after kidney transplantation, loss of graft function reportedly progresses slowly. Some reports describe successful long-term graft survival after treatment for ITG recurrence. Therefore, kidney transplantation was not contraindicated for ITG [6].
Our patient with ITG experienced immediate recurrence after kidney transplantation. His recurrent ITG did not respond to steroid pulse therapy. Although plasmapheresis was effective initially, renal function deteriorated gradually and maintenance hemodialysis started 9 months after transplantation. We herein report the clinical course of this case and review the relevant literature.
Case Report
A 55-year-old man who developed nephrotic syndrome was diagnosed with ITG, proven by kidney biopsy in July 2004. Although he underwent steroid pulse therapy, his renal function deteriorated gradually. Hemodialysis was initiated in October 2006. Except for ITG, he had no other medical history. He was introduced to our hospital for pre-transplant screening in 2008. Two HLA-A/B mismatches with a donor candidate and another in HLA-DR were found. Lymphocyte cytotoxicity test was negative. Preoperative evaluation of his general condition was sufficient to conduct transplant surgery.
In May 2009, he was admitted to our hospital for living-donor ABO incompatible kidney transplantation (ABOI-KTx) from his wife. The patient’s blood type was O; the donor’s blood type was A. According to our hospital’s protocol for ABOI-KTx, the patient was administered 200 mg of anti-CD20 antibody, rituximab (RXM) on preoperative day 6. Also, tacrolimus (0.1 mg/kg/day) and mycophenolate mofetil (2 g/day) were started on preoperative day 6. In addition, 20 mg of anti-CD25 antibody, basiliximab (BXM) was administered twice on POD 0 and 4. The titer of anti-blood group A antibodies (Anti-A Ab) before admission was as low as 8 in both of IgG and IgM. Therefore, preoperative plasmapheresis was not done. The operation was conducted without any event. Immediate renal graft function was achieved. The serum creatinine (s-Cr) level decreased to 1.4 mg/dL at a nadir. Sufficient urine volume was maintained for a few days. On POD 4, proteinuria (3.11 g/day) was detected with hematuria (≥100 red blood cells in a high power field). On POD 11, s-Cr level was elevated to 2.52 mg/dL. A transplanted kidney biopsy was conducted, revealing acute antibody-mediated rejection (aAMR). Plasmapheresis with steroid pulse therapy was performed for aAMR. Then s-Cr level was lowered and the amount of proteinuria also decreased to 0.85 g/day, while the microscopic hematuria persisted. Although the biopsy showed aAMR and electron deposition, the latter of which suggested ITG recurrence, the main cause of elevation of s-Cr level was regarded as a result of aAMR at that time.
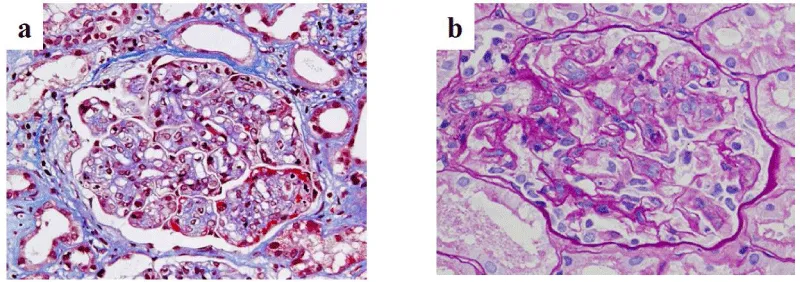
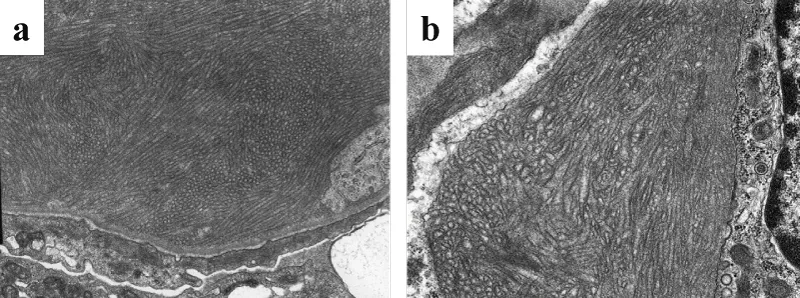
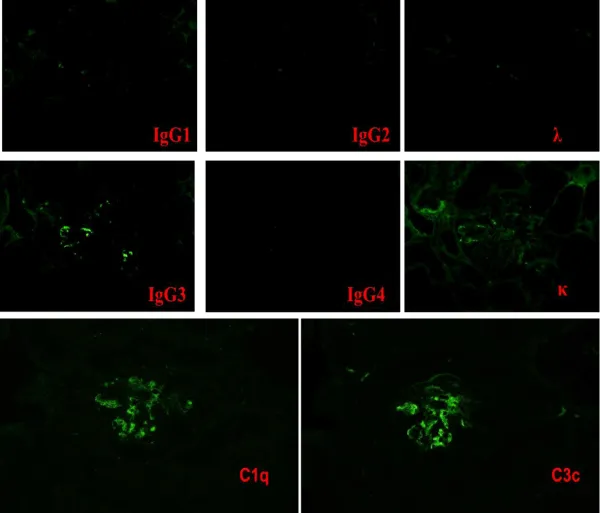
On POD 22, the s-Cr level increased again. A second renal biopsy was performed, the diagnosis of which was ITG recurrence. MPGN-like appearance was detected using light microscopy, which was similar to specimen of native kidney with ITG (Figure 1). Electron microscopy proved the presence of glomerular deposits of 30–50 nm diameter microtubules, confirming the definitive diagnosis of ITG recurrence (Figure 2b). Those deposits were similar to the patient’s native kidney (Figure 2a). Immunostaining was positive for IgG3κ as well as complement C3 and C1q (Figure 3). No evidence of rejection was found. Then steroid pulse therapy and plasmaphereses were performed for ITG recurrence. At this time, serum protein electrophoresis showed a monoclonal peak of IgGκ. Hypocomplementemia was also evident, in which C3 was reduced to 67.8 mg/dL (normal range, 80–160 mg/dL). Total serum hemolytic complement (CH50) and C4 were normal, 33.8 U/mL (25.0–48.0 CH50/ml) and 17.1 mg/dL (15–40 mg/dL), respectively. After this second round of treatment, s-Cr level fell to 1.5 mg/dL associated with improved clinical parameters of hematuria (40–50/HF) and proteinuria (0.43 g/day). The patient was discharged on POD 49.
The patient was readmitted to the hospital with a complaint of general malaise and systemic edema only 16 days after the discharge. s-Cr level was again elevated to 2.5 mg/dL, and microscopic hematuria continued with 100 red blood cells in a high power field. The amount of urinary protein rose again up to 4.5 g/day. A third kidney biopsy and plasmaphereses were done immediately. Neither urine test results nor s-Cr levels improved this time. Proteinuria continued to increase up to around 6–7 g/day. Despite periodic plasmaphereses (2–3/week), the patient’s general condition deteriorated with the development of systemic edema and ascites retention that was associated with s-Cr elevation. Eventually, hemodialysis was re-instituted 9 months after transplantation.
In this renal transplant patient, ITG recurred in the renal graft soon after kidney transplantation. Thereafter, M proteinemia and hypocomplementemia were detected. Although RXM and BXM were administered in accordance with our protocol for ABOI-KTx, and although plasmaphereses were also frequently conducted postoperatively, the transplanted kidney function deteriorated progressively. Finally, the recurred ITG advanced to a stage where it did not effectively respond to any conventional treatment for aAMR.
Discussion
This rare disease, ITG that is found in fewer than 1% of all kidney biopsies has characteristic electron-dense deposits with a microtubular structure in the glomeruli visualized using electron microscopy, but appearing negative with Congo red staining [2]. Actually, ITG can be distinguished pathologically from other types of glomerulopathies accompanied with fibrillary deposits, such as fibrillary gromerulonephritis, lupus nephritis, cryoglobulinemia and light-chain deposition disease. The deposits in ITG appear as hollow, regularly arranged microtubulars, with diameter greater than 30 nm [7].
Clinically, ITG can engender proteinuria (often in the nephrotic range), microscopic hematuria and often hypertension, which might result in end stage renal disease necessitating renal replacement therapy in the long run [2]. ITG and other deposition diseases are well known for their frequent recurrences after KTx [8]. Although the rate of ITG recurrence is as high as 47–64% [7,9], kidney transplantation in patients with ITG is not contraindicated because of the presence of successful cases [10].
Korbet et al. reported a successful case of KTx for ITG [10]. Azathioprine, prednisolone and anti-lymphocytoglobulin were used as immunosuppressive agents. The graft function was stable for about five years with no recurrence. Neither monoclonal proteinemia nor hypocomplementemia was detected. In the other three cases, ITG recurred within 1.5–19 months [11–13]. Prednisolone, cyclosporine, azathioprine, tacrolimus, mycophenolate mofetil and thymoglobulins were administered as immunosuppressants. Prednisolone pulse therapy, cyclophosphamide, rituximab and plasma exchange were added as treatment for recurrence. After these therapies, each graft function recovered and lasted more than 1 year. Neither monoclonal proteinemia nor hypocomplementemia was detected in these cases.
Korbet et al. reported another case which resulted in graft loss [10]. In this case, ITG recurred about 4.5 years after transplantation. Although the treatment for recurrence was initially effective, the graft function deteriorated gradually within a year, with no evidence of monoclonal proteinemia or of hypocomplementemia. Although immunostaining was positive for IgG, λ, κ and C3 IgG subclass were not described in their report.
In our case, ITG recurred within a month and caused graft loss at the earliest timing after transplant when compared with previous reports, i.e. only 9 months after transplantation. Comparison to other cases [11-14], revealed that IgG3 subclass and hypocomplementemia were detected only in our case (Table 1). Although no report described the association between IgG subclasses and ITG recurrence, IgG3 subclass is well known to have strong affinity for complement and inducement of its activation [15]. The cause of this rapid recurrence might be the presence of monoclonal paraproteinuria which sometimes indicates the activity of ITG, and associated with an activity of ITG at the time of transplantation. Since complications of ITG are localized only in kidneys, detecting activity of ITG is difficult to know especially in ESRD patients.
The treatment for ITG recurrence after KTx has not been determined. Plasmapheresis, steroids, cyclophosphamide, mycophenolate mofetil (MMF) and RXM have been reported to be effective for the treatment of recurrence of ITG in previous case studies [11-14]. Steroid pulse therapy and plasmapheresis were tried in our case, with no good result. In addition, RXM, which had been effective for a previous case, was administered just before transplantation in this case and it could not prevent ITG recurrence.
Retrospectively speaking, the presence of monoclonal proteinemia and hypocomplementemia should have been evaluated before transplantation. Further preoperative treatment, including plasmaphereses, should be considered in cases when they were detected.
In conclusion, our case demonstrated the presence of rapid and refractory recurrence of ITG in the renal graft in which almost all treatments for antibody-mediated rejection were ineffective. Our experience suggests that monoclonal paraproteinuria might play an important role in the early recurrence of ITG. Additionally, IgG subclass tests might also be useful for making a prognosis of ITG recurrence, especially a rapidly progressive one. Further studies should be conducted to verify the findings reported herein.
- Korbet SM, Schwartz MM, Rosenberg BF, Sibley RK, Lewis EJ (1985) Immunotactoid glomerulopathy. Medicine 64: 228-243. Link: https://goo.gl/DqNAcB
- Fogo A, Qureshi N, Horn RG (1993) Morphologic and clinical features of fibrillary glomerulonephritis versus immunotactoid glomerulopathy. Am J Kidney Dis 22: 367-377. Link: https://goo.gl/bdQqgE
- Iskandar SS, Falk RJ, Jennette JC (1992) Clinical and pathologic features of fibrillary glomerulonephritis. Kidney Int 42: 1401-1407. Link: https://goo.gl/WaF5J8
- Alpers CE (1992) Immunotactoid (microtubular) glomerulopathy: an entity distinct from fibrillary glomerulonephritis? Am J Kidney Dis 19: 185-191. Link: https://goo.gl/K8cwBt
- Minami J, Ishimitsu T, Inenaga T, Ishibashi-Ueda H, Kawano Y, et al. (1997) Immunotactoid glomerulopathy: report of a case. Am J Kidney Dis 30:160-163. Link: https://goo.gl/BNu9jT
- Ponticelli C, Moroni G, Glassock RJ (2011) Recurrence of secondary glomerular disease after renal transplantation. Clin J Am Soc Nephrol 6:1214-1221. Link: https://goo.gl/Md8YeK
- Rosenstock JL, Markowitz GS, Valeri AM, Sacchi G, Appel GB, et al. (2003) Fibrillary and immunotactoid glomerulonephritis: Distinct entities with different clinical and pathologic features. Kidney Int 63:1450-1461. Link: https://goo.gl/umDGPx
- Taneda S, Honda K, Horita S, Koyama I, Teraoka S, et al. (2008) Light chain deposition disease after renal transplantation. Am J Kidney Dis 52: 621-625. Link: https://goo.gl/Ayv7mt
- Pronovost PH, Brady HR, Gunning ME, Espinoza O, Rennke HG (1996) Clinical features, predictors of disease progression and results of renal transplantation in fibrillary/immunotactoid glomerulopathy. Nephrol Dial Transplant 11: 837-842. Link: https://goo.gl/fhnvCQ
- Schwartz MM, Korbet SM, Lewis EJ (2002) Immunotactoid glomerulopathy. J Am Soc Nephrol 13: 1390-1397. Link: https://goo.gl/w46x8Y
- Korbet SM, Rosenberg BF, Schwartz MM, Lewis EJ (1990) Course of renal transplantation in immunotactoid glomerulopathy. Am J Med 89: 91-95. Link: https://goo.gl/FwwQni
- Carles X, Rostaing L, Modesto A, Orfila C, Cisterne JM, et al. (2000) Successful treatment of recurrence of immunotactoid glomerulopathy in a kidney allograft recipient. Nephrol Dial Transplant 15: 897-900. Link: https://goo.gl/J5Em2y
- Sathyan S, Khan FN, Ranga KV (2009) A case of recurrent immunotactoid glomerulopathy in an allograft treated with rituximab. Transplant Proc 41: 3953-3955. Link: https://goo.gl/PmGFeo
- Nasr SH, Fidler ME, Cornell LD, Leung N, Cosio FG, et al. (2012) Immunotactoid glomerulopathy: clinicopathologic and proteomic study. Nephrol Dial Transplant 27: 4137-4146. Link: https://goo.gl/t9mZcH
- Imai H, Hamai K, Komatsuda A, Ohtani H, Miura AB (1997) IgG subclasses in patients with membranoproliferative glomerulonephritis, membranous nephropathy, and lupus nephritis. Kidney Int 51: 270-276. Link: https://goo.gl/ZckuZJ
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
