Archives of Organ Transplantation
The efficacy of SARS-CoV-2 antibody response after two dose mRNA vaccination in kidney and heart transplant recipients using a multiplex bead-based assay: Evaluating the factors affecting vaccine response
Steven J Forte1, Angela J Toepp2,3, Robert A Bray4, David A Baran5,6, Lauren T Gilgannon1, Troy Williams1, Shirui Chen7, Hooman Sadr8, Howard M Gebel4, John M Herre5,6 and Thomas R McCune7,8*
2Department of Internal Medicine, Eastern Virginia Medical School, Norfolk, VA 23501-1980, USA
3Enterprise Analytics, Sentara Healthcare, Norfolk, VA 23501, USA
4Dept of Pathology, Emory University Hospital, Rm F149, 1364 Clifton Rd NE, Atlanta, GA 30322, USA
5Division of Cardiology, Eastern Virginia Medical School, Norfolk, VA 23501-1980, USA
6Advanced Heart Failure and Transplantation, Sentara Norfolk General Hospital, Norfolk, VA 23507-1999, USA
7Division of Nephrology, Eastern Virginia Medical School, Norfolk, VA 23501-1980, USA
8Sentara Norfolk General Hospital, Kidney/Pancreas Transplant Program, Norfolk, VA 23507, USA
Cite this as
Forte SJ, Toepp AJ, Bray RA, Baran DA, McCune TR, et al. (2022) The efficacy of SARS-CoV-2 antibody response after two dose mRNA vaccination in kidney and heart transplant recipients using a multiplex bead-based assay: Evaluating the factors affecting vaccine response. Arch Organ Transplant 7(1): 001-008. DOI: 10.17352/2640-7973.000019Copyright
© 2022 Forte SJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: The extent that which immunosuppressive factors contribute to the antibody response to SARS-Cov-2 vaccination in solid organ transplant patients is being better understood. This study examined antibody formation against the spike SARS-CoV-2 protein (SA) when full vaccinations were up to 2 doses and boosters were not recommended. Immunosuppressive factors that affected the vaccine responsiveness in a cohort of 100 kidney and 50 heart transplant patients were evaluated. This study utilized a novel assay to detect antibodies against 4 different domains of the spike protein and the nucleocapsid protein (NC) of the SARS-CoV-2 virus on a multiplex, bead-based platform. Positive SARS-COV-2 antibodies (SA) response required identification of the receptor-binding domain and one of the three other spike protein domains. Prior SARS-CoV-2 infection could be determined by the presence of positive NC.
Results: 150 patients were enrolled in the study (100 kidneys; 50 heart recipients). This study was performed when the Center for Disease Control and Prevention (CDC) recommended only two doses of Pfizer/BioNTech [BNT162b2] and Moderna [mRNA-1273 SARS-CoV-2] vaccine or 1 dose of Johnson & Johnson/Janssen [Ad26.COV2.S] vaccines for full SARS-CoV-2 vaccination in transplant recipients. Patients that reported a positive COVID-19 swab or had positive NC were excluded from the review because the prior infection may impact vaccine response (n = 134).
Conclusions: SA were identified in 48/134 patients (36%); 25/46 heart (54%) and 23/88 kidney transplant patients (26%) (P = 0.0012). For the patients on prednisone therapy 25/93 responded with SA (27%) while for patients not on prednisone therapy, 23/41 responded with SA (56%) (P = 0.0012). The dose of steroids (5mg a day or greater) at the time of vaccination did not adversely affect vaccine efficacy (p = 0.054). Of the patients using antimetabolite therapy, 36/113 responded with SA (32%) while 12/21 patients not on antimetabolites responded with SA (57%) (P = .027). Time since transplant was not found to affect the rate of SA production when populations were separated by type of organ transplanted. T-cell depletion induction method, calcineurin inhibitor use, and type of SARS-CoV-2 vaccine were not found to be statistically significant.
Abbreviations
CDC: Center for Disease Control and Prevention; JJ/J: Johnson & Johnson/Janssen [Ad26.COV2.S] vaccine; M: Moderna [mRNA-1273 SARS-CoV-2] vaccine; MFI: Mean-Florescence Intensity; NC: Nucleocapsid Protein of the SARS-CoV-2; P/BNT: Pfizer/BioNTech [BNT162b2] vaccine; RBD: Receptor Binding Domain of the spike protein of SARS-CoV-2; SA: Spike Protein of the SARS-CoV-2; SOT: Solid Organ Transplant Recipients
Introduction
SARS-CoV-2 vaccinations have been instrumental in stopping the case and mortality rates of COVID-19 due to the high rates of antibody development in the general population [1-3]. Vaccination against SARS-CoV-2 for solid organ transplant candidates has been a high priority due to the risk of severe disease in immunocompromised patients post-transplant [4]. Preliminary studies have suggested the rate of antibody production in solid organ transplant patients (SOT) is far lower than that of the general population [5-7]. Each transplant center uses a unique immunosuppression protocol, so it is likely the rate of antibody production following SARS-CoV-2 vaccination is variable among centers. Previous studies in kidney and heart transplant recipients observed lower SARS-CoV-2 vaccine response rates for patients on steroids and the antimetabolite drugs (azathioprine, mycophenolate mofetil, and mycophenolic acid) [5,6,8-11]. These studies were undertaken when the Centers for Disease Control and Prevention (CDC) determined that full vaccination against SARS-CoV-2 in transplant recipients was two doses of Pfizer/BioNTech [BNT162b2] (P/BNT) and Moderna [mRNA-1273 SARS-CoV-2] (M) or 1 dose of Johnson & Johnson/Janssen [Ad26.COV2.S] (JJ/J) vaccines. It is important to understand each center’s rate of successful vaccination and patient attributes that are predictive of successful vaccination.
A novel assay for SARS-CoV-2 antibodies has been developed for the multiplex bead-based platform that is commonly used to detect donor-specific antibody development after transplantation [12]. The program undertook a study to evaluate the rate of antibodies formation against the spike SARS-CoV-2 protein (SA) in heart and kidney transplant recipients at the Sentara Norfolk General Hospital transplant center after patients completed vaccinations series determined by the CDC to be full vaccinated with up to two doses and before boosters were recommended. The study evaluated immunosuppressive factors that affected the response to the two-shot SARS-CoV-2 vaccination series.
Materials and methods
The Eastern Virginia Medical School Institutional Review Board approved this study of heart, kidney, and pancreas transplant recipients followed at the transplant programs of Sentara Norfolk General Hospital to evaluate the development of SARS-CoV-2 anti-spike antibodies (SA) after SARS-CoV-2 vaccinations (IRB # 21-04-FB-0111). Kidney transplant recipients were encouraged to start their SARS-CoV-2 vaccine series 3 months after transplantation if they were on a stable immunosuppressive regimen. Heart transplant patients were encouraged to start their SARS-CoV-2 vaccine series 2 months after transplantation if their immunosuppressive regimen was stable. A sample of 150 patients from the clinics was planned. Inclusion criteria included age of 18 years or older, having received the final dose of the vaccine between 14 and 180 days from the date of sample collection, and ability to give informed consent. Participants were recruited in May 2021 when full vaccination against SARS-CoV-2 was two doses of P/BNT and M or 1 dose of JJ/J vaccines. The last study sample was obtained by 1 July 2021 before the CDC recommended a third SARS-CoV-2 vaccine for immunocompromised transplant recipients for full vaccination on 13 August 2021. Written informed consent was obtained from each participant. A questionnaire was administered that included the date of transplant and types of organs transplanted, COVID-19 symptoms, a prior positive COVID-19 nasal swab, hospitalization due to COVID-19, type of vaccine received, dates of vaccine administration, and immunosuppressive regimen at the time of vaccination. A single sample, 10cc, of blood was obtained. Serum samples were batched and assessed for antibodies against SARS-CoV-2. SARS-CoV-2 antibody testing was performed using the COVID Plus Assay (One Lambda, Inc). This test is a semi-quantitative assay and evaluates antibodies against 4 spike proteins [Full spike, S-1 segment, S-2 segment, and receptor-binding domain (RBD)] and the NC. Any identified SARS-CoV-2-associated antibodies were reported as mean-florescence intensity (MFI). Positive antibody response to the SARS-CoV-2 vaccination was determined when the MFI of the RBD exceeded 5000 MFI and there was at least one other spike protein with adequate MFI (SA) [Full Spike and S2 MFI>=5000; S1 MFI>=7500]. Positivity antibody development against the NC protein was determined by an MFI >= 5,000 [12].
The subjects’ electronic health records were reviewed for induction immunosuppression at transplantation and any recent immunosuppressive therapies used to treat acute cellular and/or antibody-mediated rejection.
Categorical data for SARS-CoV-2 SA production (positive/negative) and NC identification (positive/negative) were assessed using a two-sided Chi-Square test or Fisher’s exact test where appropriate. Odds ratios were also calculated. Continuous data for antibody MFI, length of time from transplant based on the type of transplant, and length of time from transplant concerning SARS-CoV-2 spike protein antibody production was evaluated using two-tailed unpaired student’s t-tests. P-values less than 0.05 were considered statistically significant. Multivariable logistic regression was performed controlling for: sex, vaccine, induction therapy type, organ transplanted, time to transplant, and several immunosuppressives prescribed. P-values of less than 0.05 were considered significant. All analyses were performed using SAS 9.4 software (SAS Institute Inc., Cary, NC) and GraphPad Prism 9.3.1 (GraphPad Software Inc., La Jolla, CA).
This study was funded by the Sentara Clinical Research Institute, Sentara Healthcare, Norfolk Virginia.
Results
Participants
Patients followed in the transplant clinics that had previously expressed interest in having post-vaccination SARS-CoV-2 antibody titers measured were invited to participate in the study. The kidney/pancreas transplant clinic enrolled 100 patients and the heart transplant clinic enrolled 50 patients. Patients with combined heart and kidney transplants were considered kidney transplant patients because the kidney transplant service directed the immunosuppressive medications.
To assess the response to the SARS-CoV-2 vaccine without the influence of prior COVID-19 infection, study participants were evaluated for prior infections. The questionnaires identified 20 patients that reported symptoms of COVID -19 infections of those 12 reported a positive COVID-19 swab. When NC titers were compared only the 12 COVID-19 swab-positive patients were felt to have had prior COVID-19 infection and were removed from the study population. Four additional patients who did not report experiencing COVID-19 symptoms or have had a positive COVID-19 swab were found to have a positive NC were removed. Three of the four patients with positive NC were also positive SA and on review of their charts two had a COVID-19 infection before their transplantations, one had a COVID-19 infection after transplant and one patient had been transplanted before the COVID-19 pandemic and may have had an asymptomatic SARS-CoV-2 infection.
The final study population was 134 divided into 88 kidney recipients and 46 heart recipients. (Table 1). Within the study population of 134 patients without prior SARS-C0V-2 exposure, positive SA was identified in 48 patients (36%). SA were identified in 25 heart (54%) and 23 kidney transplant patients (26%) (p = 0.0012). Heart transplant recipients were more than 3.364 times more likely to develop SA after vaccination. Heart transplant recipients were more than 3 times more likely to develop SA after vaccination. When the time from transplant and SARS-CoV-2 vaccination was evaluated organ-specific trends were lost. The average time from transplant to vaccination for the entire population was 5.69 years. On average the heart transplant recipients were 7.02 years from transplant and kidney recipients were 5.00 years between transplant and SARS-CoV-2 vaccination (p = 0.82). In the entire group patients that responded to the SARS-CoV-2 vaccination were on average 7.56 years from transplant while those who did not develop SA were 4.65 years from transplant (p = 0.011). When the two organ types have separated the effect of time on vaccination efficacy was lost. Heart transplant recipients that responded to the vaccine were an average of 8.12 years from transplant and those that failed to develop SA were 5.71 years from transplant (p = 0.228). For kidney transplant recipients the average time from transplant was 6.96 years for responders and 4.31 years for those who failed to develop SA (p = 0.078).
There was no difference in vaccine responsiveness between the three vaccines. Fifty-five patients received the PBNT (41%), 78 received M (58%), and 1 received JJ/J (<1%). Development of SA was observed in 21 patients who received the PBNT vaccine (38%), 27 patients who received the M vaccine (35%), and 0 patients who received the JJ/J vaccine (0%) (p = 0.673).
The age of the patient at the time of vaccination was not associated with a difference in vaccine response rates as a group or in each organ type. Gender was not associated with a difference in vaccine response rates (p = 0.085).
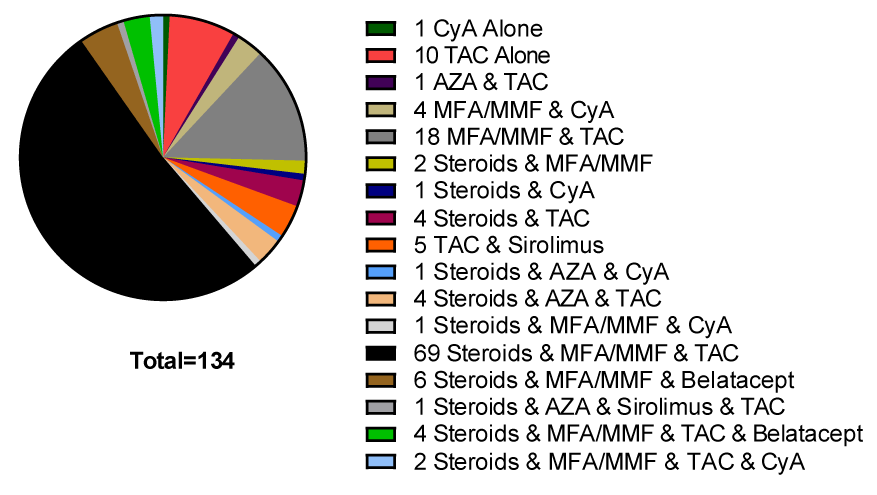
The immunosuppression medications utilized at the time of vaccination can be found in Figure 1.
Data review
The effect of immunosuppression on SARS-CoV-2 vaccination response rates was evaluated in the patients without prior exposure to the SARS-CoV-2 virus (Table 2). Immunotherapy at the initiation of immunosuppression was evaluated. Induction T-cell depletion therapy was utilized in 100 patients, with 73 having received anti-thymocyte globulin(rabbit), 25 received basiliximab, and 2 muromonab-CD3. There was no association between induction immunosuppression and vaccine response (p = 0.28).
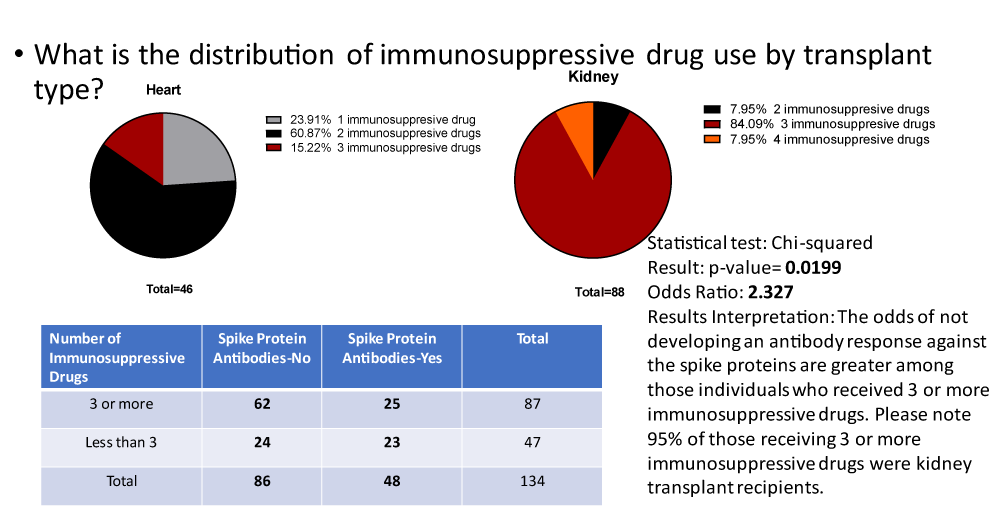
Immunosuppression therapy at the time of SARS-CoV-2 vaccination was evaluated to evaluate the effects on the development of SA. For patients on three or more immunosuppressive drugs, 28.7% (25 of 87) responded with SA but for those on less than three immunosuppressive drugs, 29% (23 of 47) responded with SA (p = 0.019). When the two types of transplant patients were evaluated for the number of immunosuppressive medications utilized, 87% of heart transplant recipients were on two or fewer drugs while 92% of kidney recipients were on three or more drugs (Figure 2).
When the immunosuppressive drugs were separated as independent agents the agents associated with a negative effect on SARS-CoV-2 vaccination became evident. Prednisone was the most common corticosteroid used but all corticosteroids were included in the group as ‘steroids’. The drugs mycophenolate mofetil, mycophenolate sodium, and azathioprine were considered together as ‘antimetabolite’ agents. The drugs cyclosporin A and tacrolimus were considered together as ‘calcineurin inhibitors’. Transplant patients not using steroids were more than 3.476 times more likely to develop SA after SARS-CoV-2 vaccination. Of the 41 patients not on steroids 23 (56%) developed SA after vaccination compared to only 25 of 93 patients (27%) on steroids (p = 0.001). There was not a dose-effect related to steroid dose at the time of vaccination. Patients on steroids that responded to the SARS-CoV-2 vaccine had the same average daily dose as those that failed to respond (p = 0.053). Transplant patients avoiding antimetabolite immunosuppression were more than 2 times more likely to develop SA after SARS-CoV-2 vaccination. Of the 113 patients on antimetabolite therapy, 36 developed SA (32%) while 12 of the 21 patients (57%) not on these drugs developed SA (p = 0.28). There was no benefit from avoiding calcineurin inhibitors. None of the patients not on calcineurins developed SA, while 48 of 129 (38%) patients on calcineurins developed SA (p = 0.157). The use of sirolimus (n = 6) and belatacept (n = 8) could not be evaluated due to the low numbers of patients on these drugs.
Multivariable logistic regression was utilized to evaluate the patient factors that are associated with a positive response to SARS-CoV-2 vaccination (Table 3). The one patient that received JJ/J was removed from this statistical analysis. The entire group was evaluated, and the two organ transplant groups were not separated in this analysis. The variables gender, vaccine type, induction T-cell depletion, type of organ transplant received, and the number of immunosuppression drug types at the time of vaccination were evaluated. The types of drugs were not separated in this analysis. The age of the patient at the time of vaccine was not included because age and time from transplant to vaccination are closely associated. The only factor that was associated with vaccine response rate was time for transplant to vaccination for the entire cohort (p = 0.022).
Discussion
This study was able to successfully employ a multiplex bead-based assay technique to determine evidence of prior COVID-19 infection as defined as a positive NC and response to SARS-CoV-2 vaccination with the development of SA. The use of this technic allowed for the exclusion of patients infected with the SARS-CoV-2 virus before the vaccine and assess SA development in response to the vaccine alone. It has been shown in solid organ transplant recipients that prior COVID-19 infections resulted in higher titers response to SARS-CoV-2 vaccination [6]. Many studies of SARS-CoV-2 vaccination in solid organ transplant recipients have failed to exclude previously COVID-19 infected patients [7-10,13-15]. Failing to exclude previously COVID-19 infected transplant patients may overestimate vaccine responsiveness. One study to evaluate the response to the Pfizer vaccine in kidney transplant patients excluded patients that were found to have positive NC [5]. Another study excluded solid organ transplant patients with a positive COVID-19 nasopharyngeal swab before vaccination with the Moderna [6]. This study excluded heart and kidney transplant patients with both a positive COVID-19 nasopharyngeal swab and/or a positive NC antibody. This double exclusion from analysis allowed for the removal of two patients who had incorrectly completed their questionnaires and were found to have positive nasopharyngeal swabs before vaccination. One patient has questioned and found to have had a viral illness before the transplant but was not tested for COVID-19. One patient with a positive NC may have had an asymptomatic COVID-19 infection because they were transplanted many years before the COVID-19 pandemic and were without any report of COVID-19 symptoms or a positive nasopharyngeal swab.
This study failed to observe an association between the use or type of t-cell depletion as induction immunotherapy and vaccine responsiveness. These findings counter a study evaluating the effectiveness of the Moderna vaccine in kidney transplant patients which excluded patients with prior COVID-19 infections and observed a decreased effectiveness in the first year after t-cell depletion therapy [16]. This study confirmed the findings of a cohort of heart and kidney transplant recipients that found no negative effect from induction t-cell depletion [9].
This study observed no benefit in the development of SA after SARS-CoV-2 vaccination from calcineurin avoidance. These results were supported by similar results in other studies that observed t-cell depletion was not associated with poor vaccine response rates [5,6,8-10].
This study found no association between vaccine responsiveness and time from transplant when the two organ transplant groups were separated using univariant analysis. When the multivariable analysis was utilized, time from transplant was associated with an improved vaccine response rate. These results were observed in a similar study that found that time from transplant for all the organ types of the study affected vaccine response rates [9]. The time from the transplant can also be a can a marker of the reconstitution of the native immune system after the induction immunosuppression used at transplantation.
In an initial review of these results, it seems that heart transplant patients had a better response to the vaccine than did the kidney recipients. The different response rates between the groups are explained by the fact that 87% of heart transplant recipients were on two or fewer drugs while 92% of kidney recipients were on three or more drugs. Kidney transplant patients were more likely to be on steroids than heart transplant patients. This is the exact opposite results of a study that found less response to the SARS-CoV-2 vaccine in heart transplant patients compared to the kidney transplant patients of the study [9]. In that studied heart transplant, patients were on more immunosuppressives than their kidney compatriots while this study observed the opposite with most heart transplant recipients on 2 or fewer immunosuppressant drugs. Both these studies agree that avoidance of steroids and antimetabolites results in higher vaccine response rates.
The improved vaccine response in patients not using steroids observed in this study supports other studies [5,6,8-10]. When the odds ratios are compared in this study, steroid avoidance is the most important factor for successful SARS-CoV-2 vaccine responsiveness. The results of this study did not support a study that suggested lower doses of steroids resulted in better vaccine responsiveness [5]. This study was only able to observe a benefit to vaccine responsiveness when steroids were completely avoided.
This study observed that patients not receiving mycophenolate mofetil, mycophenolate sodium and azathioprine were more likely to respond to vaccination with the production of SA. The improved vaccine response in patients not using antimetabolite immunosuppression observed in this study supports the findings of other studies [5,6,8-10,13,15].
This study was conducted when the CDC recommended only two doses of the P/BNT and M vaccine and one dose of the JJ/J. The results were compared to studies conducted during the same period when the CDC’s initial vaccination recommendations were being followed [5-11,13,15]. The last study sample from this study was obtained by 1 July 2021 before the CDC recommended a third SARS-CoV-2 vaccine for immunocompromised transplant recipients for full vaccination on 13 August 2021 and the SARS-CoV-2 vaccine booster dose was recommended on 21 October 2021. Therefore these studies will not be reproduced, and all studies conducted during this period are vital to our understanding of the effect of immunosuppression on organ transplantation. A subsequent study reported that after a third SARS-CoV-2 vaccine dose development on SA increased from 40% to 68% yet there was no difference in response related to current immunosuppressive [17]. Future deployment of new vaccines should include immunosuppressed patients at the development stage to ensure adequate initial dosing can be recommended before implementation to avoid the delays experienced with the introduction of the SARS-CoV-2 vaccine program.
Conclusion
This small study of kidney and heart transplant recipients showed that only 40.67% of patients responded with the development of spike antibodies after SARS-CoV-2 vaccination. The type of mRNA SARS-CoV-2 vaccine administered was not associated with a different SA response rate. Avoidance of steroids and antimetabolite therapy at the time of vaccination were independently associated with a higher SA response rate. Employing lower doses of steroids did not improve the SA response rate The use of calcineurin inhibitors did not affect SA response after SARS-CoV-2 vaccination. T-cell depletion induction immunotherapy use at the time of vaccination was not found to significantly affect antibody response. Time from transplantation was not associated with lower vaccine SA response rates when each transplant type was compared independently. This study used more restrictive criteria to exclude participants with COVID-19 infection before SARS-CoV-2 vaccination and employed a novel antibody analysis; despite these differences, this study confirms the results of other previously published reports.
Author contributions
SJ: Collected data, analyzed data, and principally wrote the paper
AT: Analyzed data and wrote the paper
RB: Designed the study, performed SARS-CoV-2 analysis, analyzed data, and wrote the paper
DB: Designed the study, analyzed the data and wrote the paper
LG: Collected the data and analyzed the data
TW: Collected the data and analyzed the data
SC: Collected the data and analyzed the data
HS: Designed the study and analyzed the data
HG: Designed the study, analyzed the data, and wrote the paper
JM: Performed SARS-CoV-2 analysis, analyzed data, and wrote the paper
TM: Designed the research/study, performed research/study, collected data, analyzed data and wrote the paper.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N Engl J Med. 2020 Dec 31;383(27):2603-2615. doi: 10.1056/NEJMoa2034577. Epub 2020 Dec 10. PMID: 33301246; PMCID: PMC7745181.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021 Feb 4;384(5):403-416. doi: 10.1056/NEJMoa2035389. Epub 2020 Dec 30. PMID: 33378609; PMCID: PMC7787219.
- Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B, Offergeld K, Scheper G, Taylor KL, Robb ML, Treanor J, Barouch DH, Stoddard J, Ryser MF, Marovich MA, Neuzil KM, Corey L, Cauwenberghs N, Tanner T, Hardt K, Ruiz-Guiñazú J, Le Gars M, Schuitemaker H, Van Hoof J, Struyf F, Douoguih M; ENSEMBLE Study Group. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N Engl J Med. 2021 Jun 10;384(23):2187-2201. doi: 10.1056/NEJMoa2101544. Epub 2021 Apr 21. PMID: 33882225; PMCID: PMC8220996.
- Negahdaripour M, Shafiekhani M, Moezzi SMI, Amiri S, Rasekh S, Bagheri A, Mosaddeghi P, Vazin A. Administration of COVID-19 vaccines in immunocompromised patients. Int Immunopharmacol. 2021 Oct;99:108021. doi: 10.1016/j.intimp.2021.108021. Epub 2021 Jul 28. PMID: 34352567; PMCID: PMC8316069.
- Grupper A, Rabinowich L, Schwartz D, Schwartz IF, Ben-Yehoyada M, Shashar M, Katchman E, Halperin T, Turner D, Goykhman Y, Shibolet O, Levy S, Houri I, Baruch R, Katchman H. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021 Aug;21(8):2719-2726. doi: 10.1111/ajt.16615. Epub 2021 May 7. PMID: 33866672; PMCID: PMC8250589.
- Hall VG, Ferreira VH, Ierullo M, Ku T, Marinelli T, Majchrzak-Kita B, Yousuf A, Kulasingam V, Humar A, Kumar D. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am J Transplant. 2021 Dec;21(12):3980-3989. doi: 10.1111/ajt.16766. Epub 2021 Aug 4. PMID: 34347934; PMCID: PMC8441872.
- Boyarsky BJ, Chiang TP, Ou MT, Werbel WA, Massie AB, Segev DL, Garonzik-Wang JM. Antibody Response to the Janssen COVID-19 Vaccine in Solid Organ Transplant Recipients. Transplantation. 2021 Aug 1;105(8):e82-e83. doi: 10.1097/TP.0000000000003850. PMID: 34098566; PMCID: PMC8298284.
- Marinaki S, Degiannis D, Roussos S, Xagas E, Tsoutsoura P, Adamopoulos S, Sypsa V, Chaidaroglou A, Pavlopoulou ID, Hatzakis A, Boletis IN. Head-to-Head Comparison of Response Rates to the Two mRNA SARS-CοV-2 Vaccines in a Large Cohort of Solid Organ Transplant (SOT) Recipients. Vaccines (Basel). 2022 Jan 25;10(2):190. doi: 10.3390/vaccines10020190. PMID: 35214649; PMCID: PMC8876597.
- Marion O, Del Bello A, Abravanel F, Faguer S, Esposito L, Laure Hebral A, Bellière J, Izopet J, Kamar N. Predictive Factors for Humoral Response After 2-dose SARS-CoV-2 Vaccine in Solid Organ Transplant Patients. Transplant Direct. 2021 Dec 23;8(1):e1248. doi: 10.1097/TXD.0000000000001248. PMID: 34966837; PMCID: PMC8710345.
- Benotmane I, Gautier-Vargas G, Cognard N, Olagne J, Heibel F, Braun-Parvez L, Martzloff J, Perrin P, Moulin B, Fafi-Kremer S, Caillard S. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021 Jun;99(6):1498-1500. doi: 10.1016/j.kint.2021.04.005. Epub 2021 Apr 20. PMID: 33887315; PMCID: PMC8055921.
- Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, Garonzik-Wang JM. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA. 2021 Jun 1;325(21):2204-2206. doi: 10.1001/jama.2021.7489. PMID: 33950155; PMCID: PMC8100911.
- Bray RA, Lee JH, Brescia P, Kumar D, Nong T, Shih R, Woodle ES, Maltzman JS, Gebel HM. Development and Validation of a Multiplex, Bead-based Assay to Detect Antibodies Directed Against SARS-CoV-2 Proteins. Transplantation. 2021 Jan 1;105(1):79-89. doi: 10.1097/TP.0000000000003524. PMID: 33273320.
- Toniutto P, Falleti E, Cmet S, Cussigh A, Veneto L, Bitetto D, Fornasiere E, Fumolo E, Fabris C, Sartor A, Peressutti R, Curcio F, Regattin L, Grillone L. Past COVID-19 and immunosuppressive regimens affect the long-term response to anti-SARS-CoV-2 vaccination in liver transplant recipients. J Hepatol. 2022 Mar 10:S0168-8278(22)00122-2. doi: 10.1016/j.jhep.2022.02.015. Epub ahead of print. PMID: 35283215; PMCID: PMC8908852.
- Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, Garonzik-Wang JM. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA. 2021 Jun 1;325(21):2204-2206. doi: 10.1001/jama.2021.7489. PMID: 33950155; PMCID: PMC8100911.
- Rashidi-Alavijeh J, Frey A, Passenberg M, Korth J, Zmudzinski J, Anastasiou OE, Saner FH, Jahn M, Lange CM, Willuweit K. Humoral Response to SARS-Cov-2 Vaccination in Liver Transplant Recipients-A Single-Center Experience. Vaccines (Basel). 2021 Jul 4;9(7):738. doi: 10.3390/vaccines9070738. PMID: 34358154; PMCID: PMC8310292.
- Cucchiari D, Egri N, Bodro M, Herrera S, Del Risco-Zevallos J, Casals-Urquiza J, Cofan F, Moreno A, Rovira J, Banon-Maneus E, Ramirez-Bajo MJ, Ventura-Aguiar P, Pérez-Olmos A, Garcia-Pascual M, Pascal M, Vilella A, Trilla A, Ríos J, Palou E, Juan M, Bayés B, Diekmann F. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021 Aug;21(8):2727-2739. doi: 10.1111/ajt.16701. Epub 2021 Aug 4. PMID: 34036720; PMCID: PMC8222867.
- Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three Doses of an mRNA COVID-19 Vaccine in Solid-Organ Transplant Recipients. N Engl J Med. 2021 Aug 12;385(7):661-662. doi: 10.1056/NEJMc2108861. Epub 2021 Jun 23. PMID: 34161700; PMCID: PMC8262620.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
