Journal of Cardiovascular Medicine and Cardiology
Serum from patients with chest pain and significant atherosclerosis resulted in macrophage cholesterol accumulation
Marwan Dawood1,2*, Nina Volkova2, Dima Namouz1, Michael Aviram2 and Tony Hayek1,2
2The Lipid Research Laboratory, Ruth & Bruce Rappaport Faculty of Medicine, Technion - Israel Institute of Technology, Haifa, Israel
Cite this as
Dawood M, Volkova N, Namouz D, Aviram M, Hayek T (2020) Serum from patients with chest pain and significant atherosclerosis resulted in macrophage cholesterol accumulation. J Cardiovasc Med Cardiol 7(3): 221-225. DOI: 10.17352/2455-2976.000142Aims: The aim of our study was to analyze the effect of serum derived from patients with chest pain, with or without significant atherosclerosis, on J774A.1 macrophages cholesterol metabolism.
Methods: Thirty-nine patients with chest pain underwent CT Coronary Angiography (CTCA) to assess atherosclerosis. They were divided into three groups (n=13 for each group), according to extent of Coronary Artery Disease (CAD): No disease (NCAD), Non-significant (NSCAD), or significant CAD (SCAD).
Results: Serum total cholesterol level was similar in all three groups, whereas the SCAD group had the lowest serum HDL level and highest serum triglyceride levels, compared with the other groups.
The patient’s serum (30µl) was incubated with J774 macrophages for 18h. Cellular cholesterol mass was found to be significantly higher by 44 percent in SCAD patients compared to NCAD patients, and by 23 percent compared to NSCAD patients.
In parallel, we observed a significant enhanced cholesterol biosynthesis rate by 53 percent in macrophages treated with serum from the SCAD patients, as compared to NCAD patients, or by 17 percent when compared to the NSCAD patients.
In accordance with the above results, HMGCR (the rate limiting enzyme in cholesterol biosynthesis) expression was significantly upregulated in macrophages treated with serum from SCAD patients, in comparison to NCAD or NSCAD patients.
Conclusion: These results clearly demonstrate high macrophage atherogenicity for serum harvested from patients with significant atherosclerosis.
Abbreviations
CTCA: CT coronary angiography; SCAD: Significant Coronary Artery Disease; NSCAD: Non-Significant Coronary Artery Disease; HMGCR: Hydroxy Methyl Glutaryl Coenzyme Reductase; CRP: C Reactive Protein; LDL: Low Density Lipoprotein; HDL: High Density Lipoprotein; IMT: Intima Media Thickness; PBS: Phosphate Buffer Saline; DMEM: Dulbecco’s Modified Eagle’s Medium; PCEC: Percentage of Cholesterol Efflux Capacity; BMI: Body Mass Index
Introduction
Atherosclerosis is a progressive disease and the leading cause of morbidity and mortality worldwide [1]. A crucial early step in atherosclerosis development is infiltration of blood monocytes from the circulation into the arterial wall [2], where they differentiate into macrophages. These intimal macrophages can take up oxidatively modified lipoproteins at enhanced rate, leading to lipid accumulation and foam cells formation, the hallmark of early atherogenesis [2,3]. Cholesterol accumulation in macrophages can also result from increased cholesterol biosynthesis, and/or decreased cholesterol efflux from the cells [3]. Experimental data support a role of monocytes in acute coronary syndromes and outcome post-infarction[4]. Indeed, macrophages, C-Reactive Protein (CRP), and complement and oxidized Low-Density Lipoprotein (LDL) were colocalized in plaque tissue from patients with unstable coronary artery disease[5]. This may increase vulnerability to plaque rupture and thrombosis.
Plasma levels of oxidized LDL are higher in patients with unstable angina [6], suggesting that antioxidants may have beneficial effects on macrophages [7] and atherosclerotic lesions. Indeed, we have shown that pomegranate juice consumption by patients with carotid artery stenosis reduced their Intima-Media Thickness (IMT), blood pressure, and LDL oxidation [8].
Observational studies have shown that LDL and High-Density Lipoprotein (HDL) cholesterol have opposing associations with risk of myocardial infarction, with LDL cholesterol being positively associated, and HDL cholesterol inversely associated [9-11].
Benjamin F Voight, et al. [12], showed that some genetic mechanisms that raise plasma HDL cholesterol do not seem to lower risk of myocardial infarction. It was previously shown that blood serum of patients with angiographically assessed coronary atherosclerosis can stimulate the accumulation of intracellular lipids (mostly free and esterified cholesterol) in primary culture of intimal smooth muscle cells of human aorta [13,14]. Thus, blood atherogenicity could be a target for anti-atherosclerotic therapy [15].
The current guidelines recommend CT Coronary Angiography (CTCA) for assessment of patients with stable angina pectoris and without changes in ECG or troponin[16].The purpose of the present study was to examine, for the first time, the atherogenicity of serum from SCAD patients, as compared to NSCAD or to NCAD patients, by analyzing their serum effects on macrophage cholesterol metabolism.
Methods
Patients
Thirty-nine patients with chest pain were admitted to the Internal Medicine Department at Rambam Health Care Campus (Haifa, Israel) for CTCA, in order to assess atherosclerosis in their coronary arteries. They were divided into three groups of 13 patients according to their results: No disease (NCAD), Non-significant (NSCAD) and significant CAD (SCAD). All patients gave written informed consent in accordance with the declaration of HELSINKI. The protocol was approved by the Rambam Health Care Helsinki Committee (protocol 0373-18-RMB).
J774A.1 macrophages
J774A.1 murine macrophage-like cell line was obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) and maintained in a humidified incubator (370C, 5% CO2) in DMEM containing 1,000 U/L penicillin, 100 mg/L streptomycin, and 5% heat-inactivated FCS.
Macrophage cholesterol mass
The cells (1×106 cells) were incubated for 18h with 30µl of the patient’s serum. After cell wash PBS (X2), cellular lipids were extracted with hexane:isopropanol (3:2, v:v). The hexane phase was evaporated under nitrogen. The amount of cellular cholesterol was determined spectrophotometrically using commercially available kit. The remaining cells in the plates were dissolved in 0.1 M NaOH, and an aliquot was taken measuring cellular protein by the Lowry assay [17,18]. This method uses the Folin-Ciocalteu Phenol reagent and albumin for the standart curve. Results were expressed as µg cholesterol/mg cell protein.
Macrophage cholesterol biosynthesis rate
The cells (1×106 cells) were incubated for 18h with 30µl of the patients’ serum, then washed with PBS and further incubated for 3h at 37°C in DMEM supplemented with 3μCi/mL [3H]-labeled acetate (PerkinElmer). After incubation, the cells were washed twice in PBS. Cellular lipids were then extracted with hexane/isopropanol (3:2 v/v), the hexane phase evaporated under nitrogen, then lipids separated by thin-layer chromatography on silica gel plates using hexane/ether/acetic acid (130:30:1.5 v/v). Cholesterol spots were visualized by iodine vapor using an appropriate standard for identification and [3H] was counted by β-counter (Packard Tri Carb 2100TR; PerkinElmer).
The remaining cells in the plates were dissolved in 0.1 M NaOH, and an aliquot taken to measure cellular protein by Lowry assay [17]; [3H] counts were normalized to cellular protein levels and expressed as cpm/mg cell protein.
RNA extraction and real‑time PCR
Total RNA was extracted from J774A.1 macrophages with Master PureTM RNA purification kit. Thermo Scientific cDNA kit was used to extract 1 μg of cDNA. Using Absolute Blue qPCR ROX mix, products of the reverse transcription were subjected to quantitative PCR using TaqMan gene expression assays with a Rotor-Gene 6000 amplification detection system; 3-hydroxy-3-methylglutarylcoenzyme reductase (HMGCR) levels were normalized to GAPDH mRNA. Primers and probes were designed by Primer Design (South Hampton, UK).
Human serum-mediated cholesterol efflux
J774A.1 macrophages were pre-incubated with [3H]-labeled cholesterol (2.5 mCi/mL) for 1h at 37°C, followed by cell wash with PBS and further incubation for 3h at 37°C with patients’ serum (30µl). At the end of the incubation period, the media (500µl) were collected and [3H]-labels were determined by liquid scintillation counting (LSC; b-counter, Packard Tri Carb 2100TR, PerkinElmer, Waltham, MA). The remaining cells in each well were washed with PBS, solubilized overnight in 0.1 M NaOH, and the amount of [3H]-labels in 500 µl of each cell lysate determined. Total cellular [3H]-cholesterol was calculated as the sum of the radioactivity in the medium and radioactivity in the lysates. Human serum-mediated cholesterol efflux corresponded to the percentage of total [3H]-cholesterol released into the medium.
Statistical analysis
Each serum was analyzed in triplicate, and each experiment was separately performed three times. Statistical analyses used one-way analysis of variance followed by the Student-Newman -Keuls test for comparing differences among multiple groups. Results are expressed as mean ± SEM of 3 separate experiments.
Results
The patient’s serum biochemical parameters
Information about the patients in each group included: age, gender, BMI, percentage HBA1C, CRP levels, number of patients with hypertension or diabetes, and those treated with statin (Table 1).
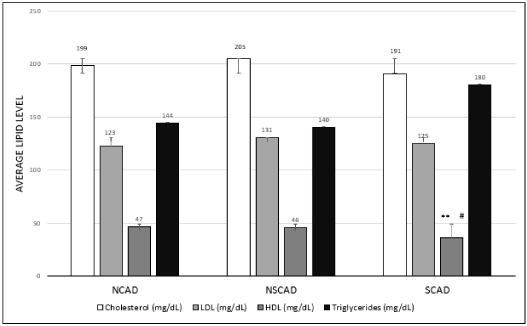
Serum total cholesterol and LDL-C levels were similar in all three groups. In contrast, the SCAD group had lower serum HDL levels compared to the NCAD and NSCAD groups (32% (p<0.02) vs. 31% (p<0.04), respectively) and increased levels of triglyceride (28% vs. 24%, p<0.24, respectively) (Figure 1).
The effect of the patient’s serum on macrophage cholesterol content
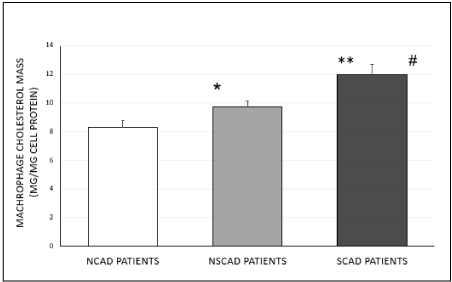
Cellular cholesterol mass was found to be significantly higher by 44% (p< 0.0003) or 23% (p< 0.008) in cells treated with serum of the SCAD patients, compared to cells treated with serum from NCAD or NSCAD patients, respectively (Figure 2A). In addition, cellular cholesterol mass was higher by 23% (p<0.01) in the NSCAD group compared to in the NCAD group (Figure 2A).
The effect of the patient’s serum on macrophage cholesterol biosynthesis
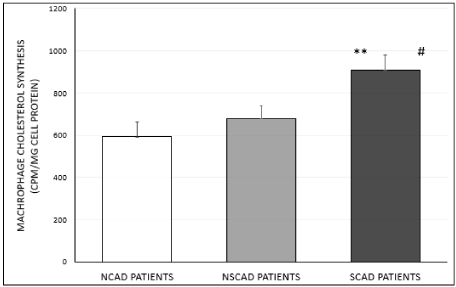
We observed a significant enhanced cholesterol biosynthesis rate of 53% (p<0.0006) and 33% (p<0.006), respectively, in cells treated with serum from the SCAD group, compared to the effect of serum from NCAD or NSCAD patients (Figure 2B). In addition, a non-significant higher cholesterol biosynthesis rate (14%) was noted in J774A.1 macrophages treated with serum from the NSCAD group compared to the NCAD group (Figure 2B).
The effect of the patient’s serum on macrophage HMGCR expression
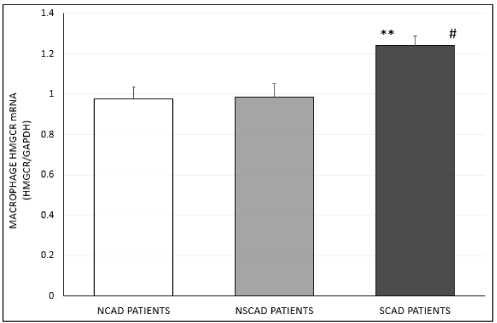
Furthermore, the enhanced macrophage cholesterol biosynthesis rate observed in cells treated with SCAD patients’ serum was found to be associated with overexpression of HMGCR mRNA levels by 27% (p<0.002) and 26% (p<0.003), respectively, in comparison to cells treated with serum from NCAD or NSCAD patients (Figure 2C). In addition, a higher (6%) but non-significant cholesterol biosynthesis rate was noted in cells treated with serum of the NSCAD compared to the NCAD group (Figure 2C).
The effect of the patient’s serum on serum-mediated cholesterol efflux from macrophages
It should be noted that there was no significant difference in the extent of serum-mediated cholesterol efflux rate from the macrophages, when comparing the three groups. Results were expressed as % serum-mediated cholesterol efflux vs. baseline (control cells incubated only with medium). Efflux rate in the SCAD group was 42±8%, compared to 40±9 and 41±7 percent, respectively, in the NSCAD and NCAD groups.
Discussion
Anti- or pro-atherogenicity of human serum is determined by its ability to attenuate or induce lipid accumulation in cultured macrophages [19]. In the current study, we clearly demonstrated, for the first time, that serum from patients with significant coronary atherosclerosis (as indicated by CT angiography), substantially increased J774A.1 macrophage cholesterol content.
Although serum cholesterol and LDL-C levels were similar in all three CTCA groups, incubation of macrophages with serum derived from atherogenic patients resulted in increased cellular cholesterol levels compared to less atherogenic patients, or to subjects with no atherosclerosis. This effect could be attributed to the significant increase in cholesterol biosynthesis rate, secondary to the observed up-regulation of HMGCR (rate limiting enzyme in cholesterol biosynthesis) expression [20]. Similarly, it was previously shown that serum taken from patients with angiographically coronary atherosclerosis induced cholesterol deposition in smooth muscle cells from human aortic intima [14]. In that study, cholesterol biosynthesis rate and HMGCR expression were not analyzed. However, the authors found in patient’s blood an LDL fraction prone to multiple modifications, including desialylation and oxidation. This LDL fraction resulted in cholesterol accumulation in cultured intima smooth muscle cells [14].
The role of HDL cholesterol in CAD is well investigated. Epidemiological studies indicate an inverse relationship between HDL cholesterol levels and risk for CAD, independent of LDL levels [10-12]. On the other hand, recent data provide evidence that high HDL levels are not always protective [11]. HDL may provide cardiovascular protection by promoting reverse cholesterol transport from macrophages [21].
Although the SCAD patients had low HDL cholesterol levels, as compared to NSCAD or NCAD patients, the ability of their serum to induce cholesterol efflux from J774A.1 macrophage was similar, suggesting the importance of investigating the functionality of the HDL, and not just HDL cholesterol concentration as a risk factor for CAD. Indeed, it was previously demonstrated that HDL from CAD patients is dysfunctional and pro-oxidant/pro-inflammatory in macrophages [22] and triggers macrophage apoptosis [23].
Associations between the presence and the severity of CAD, and the Percentage of Cholesterol Efflux Capacity (PCEC) and HDL cholesterol level in patients who underwent CTCA were investigated [24]. While PCEC was not associated with the presence or severity of CAD, total PCEC and HDL-C in patients with CAD were significantly lower than in patients without CAD.
Findings from several large studies indicate that elevated levels of triglycerides (either fasting or nonfasting) or, more specifically, triglyceride-rich lipoproteins and their remnants, are independently associated with increased risk of CVD. Increased triglyceride levels were noted in the SCAD patients, compared to NSCAD or NCAD patients. It was previously shown that triglyceride-rich lipoproteins induced cholesterol accumulation in J774A.1 macrophages [25] and THP-1 macrophages [26]. Similarly, in our study, the increased triglyceride levels in the SCAD patients could contribute to cholesterol accumulation in macrophages.
Conclusion
The SCAD patients’ serum atherogenicity can contribute to macrophage cholesterol accumulation, foam cell formation, accelerated plaque formation, and thus to a higher morbidity mortality percentage in these patients.
- Libby P (2002) Inflammation in atherosclerosis. Nature 420: 868–874. Link: https://go.nature.com/3iYPQ0n
- Xu L, Dai Perrard X, Perrard JL, Yang D, Xiao X, et al. (2015) Foamy monocytes form early and contribute to nascent atherosclerosis in mice with hypercholesterolemia. Arterioscler Thromb Vasc Biol 35: 1787-1797. Link: https://bit.ly/30aC41R
- Aviram M, Rosenblat M (2004) Paraoxonases. 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radic Biol Med 37: 1304-1316. Link: https://bit.ly/38SmZWO
- Ghattas A, Griffiths HR, Devitt A, Lip GY, Shantsila E (2013) Monocytes in coronary artery disease and atherosclerosis: where are we now? J Am Coll Cardiol 62: 1541-1551. Link: https://bit.ly/2AWmFdb
- Meuwissen M, van der Wal AC, Nisesen HW, et al. (2006) Colocalisation of intraplaque C reactive protein, complement, oxidized low density lipoprotein, and macrophages in stable and unstable angina and acute myocardial infarction. J Clin Pathol 59: 196-201. Link: https://bit.ly/32fCUxh
- Anselmi M, Garbin U, Agostoni P, Fusaro M, Pasini AF, et al. (2006) Plasma levels of oxidized-low-density lipoproteins are higher in patients with unstable angina and correlated with angiographic coronary complex plaques. Atherosclerosis 185: 114-120. Link: https://bit.ly/2AXaFrV
- Rom O, Aviram M (2016) Endogenous or exogenous antioxidants vs.pro-oxidants in macrophage atherogenicity. Curr Opin Lipidol 27: 204-206. Link: https://bit.ly/307o219
- Aviram M, Rosenblat M, Gaitini D, Nitecki S, Hoffman A, et al. (2004) Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin Nutr 23: 423-433. Link: https://bit.ly/38VWDDb
- Castelli WP (1988) Cholesterol and lipids in the risk of coronary artery disease—the Framingham Heart Study. Can J Cardiol 4: 5A–10A. Link: https://bit.ly/2OjZEnu
- Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, et al. (2007) HDL cholesterol, very low levels of LDL cholesterol and cardiovascular events. N Engl J Med 357: 1301-1310. Link: https://bit.ly/3gZHCU2
- Vergeer M, Holleboom AG, Kastelein JJ, Kuivenhoven JA (2010) The HDL hypothesis: does high-density lipoprotein protect from atherosclerosis? J Lipid Res 51: 2058–2073. Link: https://bit.ly/3fuOEiY
- Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic R, et al. (2012) Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomization study. Lancet 380: 572-580. Link: https://bit.ly/2WxXuFx
- Chazov El, Tertov VV, Orckhov AN, Lyakishev AA, Perova NV, et al. (1986) Atherogenicity of blood serum from patients with coronary heart disease. Lancet 2: 595-598. Link: https://bit.ly/2Wjs2KK
- Orekhov AN, Tertov VV, Pokrovsky SN, IYu A, Martsenyuk, et al. (1988) Blood serum atherogenicity associated with coronary atherosclerosis. Evidence for nonlipid factor providing atherogenicity of low-density lipoproteins and an approach to its elimination. Circ Res 62: 421-429. Link: https://bit.ly/2ZoNfVy
- Sobenin IA, Chistiakov DA, Bobryshev YV, Orekhov AN (2013) Blood atherogenicity as a target for anti-atherosclerotic therapy. Curr Pharm Des 19: 5954-5962. Link: https://bit.ly/2Ok9cyZ
- Dancy L, O'Gallagher K, Milton P, Sado D (2018) New NICE guidelines for the management of stable angina. Br J Gen Pract 68: 202–203. Link: https://bit.ly/2WhoIQk
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275. Link: https://bit.ly/2WkAxWa
- Zhaou C, Lei H, Chen Y, Liu Q, Li LC, et al. (2013) Enhanced SCAP glycosylation by inflammation induces macrophage foam cell formation. Plos One 8: e75650. Link: https://bit.ly/38U07pM
- Igor AS, Veronica AM, Elena VA, Pavlova XN, Möhlenkamp S, et al. (2014) Blood serum atherogenicity and coronary artery calcification. Curr Pharm Des 20: 5884–5888. Link: https://bit.ly/3iZCjp7
- Tan JME, Cook ECL, van den Berg M, Scheij S, Zelcer N, et al. (2019) Differential use of E2 ubiquitin conjugating enzymes for regulated degradation of the rate-limiting enzymes HMGCR and SQLE in cholesterol biosynthesis. Atherosclerosis 281: 137-142. Link: https://bit.ly/308rlFh
- Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, et al. (2011) Cholesterol efflux capacity, high-density lipoprotein function and atherosclerosis. N Engl J Med 364: 127-135. Link: https://bit.ly/3ft1bUj
- Sini S, Deepa D, Harikrishnan S, Jayakumari N (2017) High-density lipoprotein from subjects with coronary artery disease promotes macrophage foam cell formation: role of scavenger receptor CD36 and ERK/MAPK signaling. Mol Cell Biochem 427: 23-34. Link: https://bit.ly/3ftWvgN
- Sini S, Deepa D, Harikrishnan S, Jayakumari N (2018) Adverse effects on macrophage lipid transport and survival by high density lipoprotein from patients with coronary heart disease. J Biochem Mol Toxicol 32: e22192. Link: https://bit.ly/38UsdRT
- Norimatsu K, Kuwano T, Miura SI, Shimizu T, Shiga Y, et al. (2017) Significance of the percentage of cholesterol t efflux capacity and total cholesterol efflux capacity in patients with or without coronary artery disease. Heart Vessels 32: 30-38. Link: https://bit.ly/38VDW2Q
- Huff MW, Evans AJ, Sawyez CG, Wolfe BM, Nestel PJ (1991) Cholesterol accumulation in J774 macrophages induced by triglyceride-rich lipoproteins. Comparison of very low density lipoprotein from subjects with type III, IV and V hyperlipoproteinemias. Arterioscler Throm 11: 221-233. Link: https://bit.ly/304WJVm
- Georgopoulos A, Kafonek SD, Raikhel I (1994) Diabetic postprandial triglyceride-rich lipoproteins increase esterified cholesterol accumulation in THP-1 macrophages. Metabolism 43: 1063-1072. Link: https://bit.ly/3iZSfb8

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
