Open Journal of Orthopedics and Rheumatology
Effect of methoxatin loaded chitosan conduit on deep digital flexor tendon healing in rabbits: An animal model study
Negin Mozafari1*, Mohammad Velayati2, Azad Bahrampour3, Erfan Yarahmadi2, Roghaiyeh Neisari4 and Rahim Mohammadi2
2Department of Surgery and Diagnostic Imaging, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran
3Young Researchers and Elite Club, Sanandaj Branch, Islamic Azad University, Sanandaj, Iran
4Department of Anesthesiology, Emam Khomeini Hospital, Urmia University of Medical Sciences, Urmia, Iran
Cite this as
Mozafari N, Velayati M, Bahrampour A, Yarahmadi E, Neisari R, et al. (2020) Effect of methoxatin loaded chitosan conduit on deep digital flexor tendon healing in rabbits: An animal model study. Open J Orthop Rheumatol 5(1): 063-068. DOI: 10.17352/ojor.000029Chitosan is of great interest in regenerative medicine because of its plentiful properties, like biocompatibility, biodegradability and non-toxicity. The objective of the present study was histopathological and biomechanical survey on effect of methoxatin loaded chitosan conduit on Deep Digital Flexor Tendon (DDFT) healing in rabbit models. Eighteen healthy male white New Zealand rabbits were randomized into three groups of six animals each. In CONTROL group the DDF tenotomy was performed and the sumps were sutured. In CTN (chitosan) group the DDF tenotomy was performed and the sumps were sutured and CTN conduit was wrapped around the damaged area. In CTN/METHO (chitosan/methoxatin) group the procedure was the same as CTN group as well as local administration of 100 µL methoxatin (100 µg/Rabbit) into the CTN conduit. The histopathological assessments including inflammation, angiogenesis and collagen fibers arrangement, and biomechanical assessments were performed after 8 weeks. Histopathological observations showed that the conduit was absorbed and adhesion around the tendon was deceased in CTN and CTN/METHO groups. There were no noticeable signs of infection and tissue reaction in the granulation tissue in CTN/METHO group compared to other groups (P<0.05). Local administration of methoxatin in combination with chitosan conduit could accelerate deep digital flexor tendon healing via decrease in adhesion around the tendon with no signs of excessive tissue reaction or infection in rabbits.
Introduction
Functional association among the dynamic and the static parts of the musculoskeletal system transferring muscle contraction to the skeletal system are tendons, hence, ending up motion. Accordingly, function and motion are compromised in tendon damage ranging from acute traumatic ruptures to chronic overuse and degenerative tendinopathy. However, still with amended therapeutic approaches including non-surgical, surgical, and rehabilitation techniques, results are not satisfactory because repaired tendon tissue infrequently attains functionality the same as pre-dameged condition [1,2]. Tendon damages result in extensive morbidity, and ensued debility can remain for several months in spite of what is called proper treatment [3].
Injuries to tendons may be acute or chronic, and are produced by alone or in combination of intrinsic or extrinsic causes. In case of acute trauma, extrinsic factors prevail and in chronic cases intrinsic reasons also play a crucial role. In chronic tendon injuries there are associations between intrinsic and extrinsic factors. It has been demonstrated that in two-thirds of athletes with Achilles tendon injuries, intrinsic factors like alignment and biomechanical shortcomings play a crucial role [3].
Chitosan is a linear polysaccharide and is linked with scar-less repair of soft tissues and has been demonstrated to prevent adhesion formation within tendon repair following surgery [4,5]. Chitosan inclines to precipitate in physiologic pH that explains its effectiveness. A chitosan solution that does not precipitate in physiologic settings was recently produced [6]. Therefore, no precipitation allows it to adhere to the healing site for enough time to take effect. These properties pave the way for intimate contact between chitosan and tendon, hence, enabling guided-tissue regeneration and avoiding adhesion formation. Other biological mediators like platelet-rich plasma are administered as fluid rather than gel and are therefore more prone to diffuse from the repair site, modifying their effects. Therefore, chitosan seems to be unique among other mediators [7].
The restricted capacity of tendon for self regeneration and the overall inadequacies of existing treatment schedules have augmented the enthusiasm to develop tissue engineering approaches for tendon healing. Recently, one of the specific attitudes has been adoption of variuos types of scaffold to renew functionality of tendons and ligaments. It is important to scheme and formulate a appropriate scaffold for application in specific tissue repair, because it contacts with tissue cells, and delivers structural upkeep and regulation for successive tissue development. Towards this, more attention has been paid to the design of scaffolds for guiding cell behaviors and tissue regeneration, and the design of scaffolds should be based on knowledge learned from native tissues, such as their anatomic structures, compositions and functions [8].
The development of inflammation normally ends up the discharge of biologically active mediators to draw neutrophils, leucocytes and monocytes to the area of wound in order to invade foreign debris and microorganisms via phagocytosis. This progression results in the generation of oxygen-free radicals like hydrogen peroxide, superoxide anion, and hydroxyl anion, that their excess, leads to tissue injury where they devastate the natural antioxidant enzymes of the host like catalase, superoxide dismutase, and glutathione peroxidase. Hence, antioxidants avoid the free radicals activity and avoid cells and tissues injury, and also augment healing of wounds with or without infection [9,10].
In tissue damage, free oxygen radicals react with DNA and generate 8- hydroxyguanine (8-OHGua) that is DNA damaged product. Production of free oxygen radicals takes place uninterruptedly in cells and existence of defense systems for endogenous antioxidant protects tissues from detrimental impacts of free oxygen radicals [11]. It has been demonstrated that there are different anti-inflammatory and antioxidant free radical scavengers that bear constructive effects to avoid ischemic/reperfusion damages in tissues [12-14]. It has been indicated that methoxatin performs like an antioxidant, and it is able to prevent lipid peroxidation damage, enhance thymidine incorporation into fibroblasts and augment growth factors generation [15].
The aim of theour research was to histopathologically and biomechanically explore influence of methoxatin loaded chitosan conduit on deep digital flexor tendon healing in rabbit models. The assessments were based on macroscopic, histopathological and biomechanical criteria.
Materials and methods
Preparation and fabrication of chitosan conduit, animal grouping and procedures
CTN (85% deacetylated medium molecular weight) was supplied from Fluka, Sigma-Aldrich (USA). Acetic acid and Glycerol were purchased from Merck (Germany) and Sigma Chemical Co. (USA). The aqueous solution (1% V/V) of glacial acetic acid was prepared at first, then CTN solution (2% W/V) was prepared by adding 2 g CTN to 100 ml acetic acid (1% V/V) while stirring on a magnetic stirrer-hot plate, The solution was stirred with low heat (at 50°C) for 1 hour. The resultant CTN solution was filtered through a Whatman No. 3 filter paper (UK) to remove any un-dissolved particles and to prevent the fragility of CTN, glycerol was added in amount of 30% of the total solid weight in solution [16,17].
The conduit was fabricated according to works of other researchrs [18]. The mold with CTN solution was put in a – 80 ˚C freezer for 12 h. The frozen molds were placed at room temperature and after 5 min; the outer layers of frozen molds were removed. The frozen solutions were dried in a freeze-dryer (model Alpha 1-4 LDplus; Martin Christ, Osterode, Germany). The main drying temperature was –40 ˚C and the main drying pressure was 12 Pa for 15 hr. Then, the scaffolds were immersed into 2.00% (w/v) sodium hydroxide solution (Merck) and equilibrated for 20 min to exclude the residual acetic acid. Scaffolds were cleaned using deionized water until the rinsing solution was neutral, and then equilibrated in 0.20 mol L-1 phosphate buffered saline (pH: 7.40) for half an hour and finally scaffolds were dried at room temperature for 6 hr.19,20 The fabricated conduit was 0.20 mm thick and 3.50 ± 0.50 mm in inner diameter. All of the conduits were sterilized with formaldehyde tablets in airtight containers for 24 h.
Forty eight male New Zealand white rabbits, weighing 2.5-3.0 kg, were included into the present study. The rabbits were placed in standard cages and fed with commercial rabbit pellet and water freely. All processes were perfomed based on the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The research project received the confirmation of the Institution Ethics Committee.
The rabbits were divided into three groups of 6 animals each, randomly. They were anesthetized intramuscularly using xylazine hydrochloride 10 mg/kg (Alfasan, The Netherlands) and ketamine hydrochloride 40 mg/kg (Ketaset, Germany). The right hind limb of each rabbit was prepped and plantar skin incision was made longitudinally and DDF tenotomy was performed and tendon stumps were sutured in modified Kessler pattern using 3-0 monofilament nylon (Ethilon, Ethicon, Inc., Somerville, NJ, USA). In CONTROL group, the DDF tenotomy was performed and the sumps were sutured. In CTN group the DDF tenotomy was performed and the sumps were sutured and CTN conduit was wrapped around the damaged area. In CTN/METHO group the procedure was the same as CTN group as well as local administration of 100 µL Methoxatin (100 µg/Rabbit) into the CTN conduit.
Macroscopic assessments
Following 8 week post operation, the animals were euthanized using overdose of anesthetic agent (Thiopental 1.0 g, Biochemie, Austria) macroscopic assessments including gliding performance of tendon and formation of adhesions were done according on a scoring system explained by other investigators (Table 1) [19].
Biomechanical testing
The DDF tendons (three samples from each group) were taken and wrapped in saline-soaked gauze. They were instantly kept at -20º C until day of biomechanical testing. The suture materials were removed before initiation if testing process and the samples were thawed at room temperature. We used The TA.XTPlus Texture Analyzer mechanical test device (Stable Micro Systems, Surrey GU7 1YL, UK). The samples were attached on mechanical testing machine jaws. The original length was set to 10 mm. A 60 mm/min constant rate was selected for each sample to stretch. The load and displacement were sampled 5 times per second. Each sample was stretched to complete tensile failure. Samples were kept wet moist within testing by dropping normal saline solution on segments of tendon.
Histological assessments
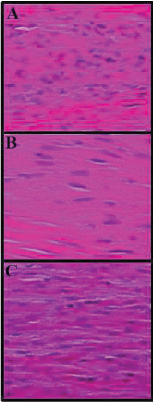
Following 8 week post operation and after macroscopic assessments the tendon samples were taken (three samples from each group) and fixed in 10% formalin solution. They were then dehydrated and embedded in paraffin wax, sectioned at 5 µm and stained with and stained with Hematoxylin and Eosin (H&E) and Masson’s trichrome stains. Images were taken using light microscope to assess inflammation, angiogenesis and collagen fibrils arrangement.
Statistical analysis
Kruskal–Wallis variance analysis were used to evaluate differences among groups. Multiple comparison tests were adopted to know differences when the P-value from the Kruskal–Wallis test statistics was significant statistically. SPSS 18 (SPSS Inc., Chicago, IL, USA) was adopted for statistical analysis. A p-value < 0.05 was set as statistical significance.
Results
Macroscopic findings
There were no signs of local infection around tendons in all experimental groups. The conduit was absorbed in the CTN/METHO and CTN groups. Remarkable peritendinous adhesions were found in the CONTROL group that needed sharp dissection for detachments. The adhesion scores in CTN/METHO and CTN groups were significantly lower than that of the CONTROL group (P=0.001) (Table 2).
Biomechanical findings
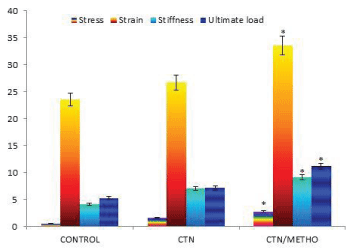
In tensile test, rupture at the repair site of samples was selected as the mode of the failure. The mechanical testing demonstrate that biomechanical indices including stress, strain, stiffness and ultimate load were significantly increased in CTN/METHO group compared to those of other groups (P=0.001) (Figure 1).
Histological findings
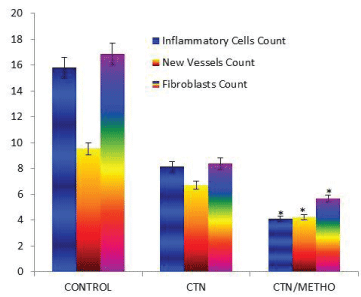
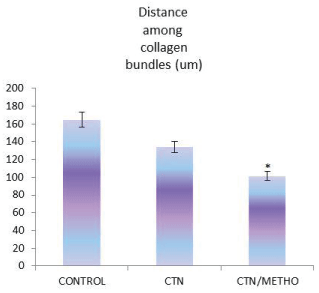
The histopathological findings of the present study demonstrated that number of ovoid-shaped tenoblasts, inflammatory cells and newly formed blood vessels were significantly decreased in number in CTN/METHO group in comparison with those of other groups (P<0.05). In H&E staining, there were fibroblasts bearing hyperchromatic and elongated nuclei between collagen fibers. Higher collagen bundles densities were observed in CTN/METHO group in comparison with those of other experimental groups (P=0.001) (Figures 2-4).
Discussion
Findings of tendon handling for repair are not satisfactory in practice. Knowledge on the healing mechanisms are adequate, however, in clinical practice little achievements have been established, and that is because the recent researches on tissue engineering of tendons are largely in preclinical level. The objective in management of tendon pathologies, acute or chronic, should be as close as to a natural tendon injury with analogous characteristics and in this regard degenerated tissues are great challenges [18-20].
Inflammation in tendon drops few days after injury, and syntheses of fibroblasts proliferation, extracellular matrix and mostly collagen type III after five day. The newly synthetized collagen fibrils are arranged in the extracellular matrix in a random fashion and after 3-4 weeks are aggregated in organized bundles. Diminution in collagen type III contents and escalation in collagen type I synthesis are considered as a key properties of tendon healing remodeling phase starting within two month post injury. In spite of immature and weak nature of collagen type III fibers and their random orientation, they are responsible for neotendon stability [20-22]. Furthermore, high expression of type I collagens and longitudinal orientation of these fibers are thought to be indispensable to get to the maximum tensile strength and accelerated tendon repair. In fact, early augmentation in collagen type I fibers post-treatment lead to early augmentation in wound tensile strength within the time in which the tendon would be at the risk of re-injury [23-24]. Therefore, the neotendons were evaluated within two months. It has been approved that extreme inflammatory response will interfere with the proliferative phase of healing and the tensile strength of the wound repair will decline as a result of scar formation [25].
Histopathological results of our study showed significant reduction in inflammatory cells was observed in CTN/METHO group, indicating beneficial effect of methoxatin loaded chitosan conduit in tendon repair.
Chitosan that is a natural polymer from deacetylation of chitin (poly-N-acetylglucosamine), has been extensively used as local dressing in wound healing because it bears antimicrobial and nontoxic, biocompatible and biodegradable characteristics [26]. At the end of the study period, the conduits were totally absorbed in CTN/METHO and CTN groups indicating biocompatibility and biodegradability of the conduit.
Adhesion formation following trauma to tendon still is challenging in practice and no satisfactory preventive measure has been established. It could be possible to formulate improved approaches to prevent adhesion formation due to advances in the understanding of the mechanisms involved [27]. Trauma is considered as the most important factor involved in adhesion formation is [28]. Key cells in tendon healing are tenocytes and tenoblasts. The actin isoform has been identified in tendons and ligaments [29]. Tenocytes that express α-smooth muscle actin are known as myofibroblasts. Stress fibers (actin microfilaments), well-developed cell-stroma attachment sites (fibronexus) and intercellular gap junctions are three essential morphological elements that define myofibroblasts [30]. Tensile forces are transferred to extracellular matrix network by fibronexus [31]. Extracellular matrix network homeostasis in tendons and ligaments is achieved by myofibroblasts that are responsible for tendon adhesions formation [32]. Efforts have been made to diminish formation of adhesion by usage of materials acting as mechanical barriers like polyethylene or silicone or by usage of pharmacological agents like ibuprofen and indomethacin, however no simple method is widely adopted [33-35].
Other investigators showed that chitosan avoids proliferation in sheath cells of tendon and production of collagen [36-37]. Chitosan prevents survival of fibroblasts that may be one of the explanations for the augmentation of gliding of tendon. The adhesion formation inhibits the gliding function of tendon and therefore, limits the range of motion of affected limb [37]. In our findings there was no peritendinous adhesions in CTN/METHO and CTN groups indicating that tendon gliding was achieved in the injured tendons.
The collagen fibers are considered as the leading structural components of tendon in charge of its mechanical strength [38-39]. The findings of biomechanical indices in the present study demonstrated higher tensile loads compared to CONTROL group. Significant augmentation in stress, strain, stiffness and ultimate load in CTN/METHO showed that Methoxatin administration ended up additional collagen deposition and remodeling.
Conclusion
Local administration of methoxatin loaded conduit improved tendon healing in rabbits. It could be concluded that use of the chitosan conduit could be of clinical benefit due to reduced peritendinous adhesion formation around injured site of tendon during repairing period and also the conduit could be used as a carrier for drug delivery to improve and accelerate tendon healing.
The authors are grateful to The Deputy Vice Chancellor for Research of the University that in part supported the study.
- Hogan MV, Bagayoko N, James R, Starnes T, Katz A, et al. (2011) Tissue engineering solutions for tendon repair. J Am Acad Orthop Surg 19: 134-142. Link: https://bit.ly/2M3cF6V
- Majewski M, Schaeren S, Kohlhaas U, Ochsner PE (2008) Postoperative rehabilitation after percutaneous Achilles tendon repair: early functional therapy versus cast immobilization. Disabil Rehabil 30: 1726-1732. Link: https://bit.ly/3aKeYX4
- Sharma P, Maffulli N (2005) Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am 87: 187-202. Link: https://bit.ly/3ptPK3j
- Wang D, Mo J, Pan S, Chen H, Zhen H (2010) Prevention of postoperative peritoneal adhesions by Ocarboxymethyl CTN in a rat cecal abrasion model. Clin Invest Med 33: E254-E260. Link: https://bit.ly/37OzIuM
- Nivedhitha Sundaram M, Deepthi S, Mony U, Shalumon KT, Chen JP, et al. (2019) CTN hydrogel scaffold reinforced with twisted poly(l lactic acid) aligned microfibrous bundle to mimic tendon extracellular matrix . Int J Biol Macromol 122: 37-44. Link: https://bit.ly/34JYsma
- Cho MH, Kim KS, Ahn HH, Kim MS, Kim SH, et al. (2008) Chitosan gel as an in situ-forming scaffold for rat bone marrow mesenchymal stem cells in vivo. Tissue Eng Part A 14: 1099-108. Link: https://bit.ly/37NlGcM
- Melamed E, Beutel BG, Robinson D (2015) Enhancement of acute tendon repair using CTN matrix. Am J Orthop (Belle Mead NJ). 44: 212-216. Link: https://bit.ly/3pm0b8U
- Khademhosseini A, Vacanti JP, Langer R (2009) Progress in tissue engineering. Sci Am 300: 64-71. Link: https://bit.ly/38AhJr1
- Houghton PJ, Hylands PJ, Mensah AY, Hensel A, Deters AM (2005) In vitro tests and ethnopharmacological investigations: wound healing as an example. J Ethnopharmacol 100: 100-107. Link: https://bit.ly/3nPQeAr
- Wang Z, Han N, Zhao K, Li Y, Chi Y, et al. (2019) Protective effects of pyrroloquinoline quinine against oxidative stress-induced cellular senescence and inflammation in human renal tubular epithelial cells via Keap1/Nrf2 signaling pathway. Int Immunopharmacol 72: 445-453. Link: https://bit.ly/3aFBYq4
- Wang Z, Li Y, Wang Y, Zhao K, Chi Y, et al. (2019) Pyrroloquinoline quinine protects HK-2 cells against high glucose-induced oxidative stress and apoptosis through Sirt3 and PI3K/Akt/FoxO3a signaling pathway. Biochem Biophys Res Commun 508: 398-404. Link: https://bit.ly/2M8g4Sa
- He K, Nukada H, Urakami T, Murphy MP (2003) Antioxidant and pro-oxidant properties of pyrroloquinoline quinone (PQQ): implications for its function in biological systems. Biochem Pharmacol 65: 67-74. Link: https://bit.ly/3aHERGN
- Hao J, Ni X, Giunta S, Wu J, Shuang X, et al. (2019) Pyrroloquinoline quinone delays inflammaging induced by TNF-α through the p16/p21 and Jagged1 signalling pathways. Clin Exp Pharmacol Physiol. Link: https://bit.ly/3mT3mDk
- Liu Z, Sun C, Tao R, Xu X, Xu L, Cheng H, et al. (2016) Pyrroloquinoline Quinone Decelerates Rheumatoid Arthritis Progression by Inhibiting Inflammatory Responses and Joint Destruction via Modulating NF-κB and MAPK Pathways. Inflammation 39: 248-256. Link: https://bit.ly/3rtebzE
- Yoneda T, Tomofuji T, Kawabata Y, Ekuni D, Azuma T, et al. (2014) Application of coenzyme Q10 for accelerating soft tissue wound healing after tooth extraction in rats. Nutrients 6: 5756-5769. Link: https://bit.ly/38zEN9A
- Jayasuriya A, Aryeai A, Jayatissa A (2013) ZnO nanoparticles induced effects on nanomechanical behavior and cell viability of CTN films. Mater Sci Eng C Mater Biol Appl 33: 3688-3696. Link: https://bit.ly/3rsdqH5
- Raisi A, Azizi S, Delirezh N, Heshmatian B, Amini K (2010) Use of CTN Conduit for Bridging Small-Gap Peripheral Nerve Defect in Sciatic Nerve Transection Model of Rat. Iran J Vet Surg 5: 89-100. Link: https://bit.ly/3mTU361
- Yousefi A, Sarrafzadeh-Rezaei F, Asri-Rezaei S, Farshid AA, Behfar M (2018) Fabrication of novel tubular scaffold for tendon repair from CTN in combination with zinc oxide nanoparticles. Vet Res Forum 9: 105-111. Link: https://bit.ly/3phfiAy
- Raisi A, Azizi S, Delirezh N, Heshmatian B, Amini K (2010) Use of CTN conduit for bridging small-gap peripheral nerve defect in sciatic nerve transection model of rat. Iran J Vet Surg 5: 89-100. Link: https://bit.ly/3o2rGUA
- Ao Q, Wang A, Cao W (2006) Manufacture of multimicrotubule CTN nerve conduits with novel molds and characterization in vitro. J Biomed Mater Res A 77: 11-18. Link: https://bit.ly/2WKZz0l
- Strickland JW (2005) The scientific basis for advances in flexor tendon surgery . J Hand Ther 18: 94-110 . Link: https://bit.ly/3mTTY2d
- Muller SA, Todorov A, Heisterbach PE, Martin I, Majewski M (2015) Tendon healing: an overview of physiology, biology, and pathology of tendon healing and systematic review of state of the art in tendon bioengineering. Knee Surg Sports Traumatol Arthrosc 23: 2097-2105. Link: https://bit.ly/38HeSNk
- Behfar M, Sarrafzadeh-Rezaei F, Hobbenaghi R, Delirezh N, Dalir-Naghadeh B (2009) Effects of uncultured adipose derived stromal vascular fraction on tendon healing in rabbits: A histological and immunohistochemical study. Iran J Vet Surg 4: 25-36. Link: https://bit.ly/3nS1xb0
- Dahlgren LA, van der Meulen MC, Bertram JE, Starrak GS, Nixon AJ (2002) Insulin-like growth factor-I improves cellular and molecular aspects of healing in a collagenase-induced model of flexor tendinitis. J Orthop Res 20: 910-919. Link: https://bit.ly/2WNXFw1
- Paul MD (2013) Barbed sutures in aesthetic plastic surgery: evolution of thought and process. Aesthet Surg J 33: 17S-31S. Link: https://bit.ly/2KWBmla
- Helary C, Desimone MF (2015) Recent advances in biomaterials for tissue engineering and controlled drug delivery . Curr Pharm Biotechnol 16: 635-645 . Link: https://bit.ly/3rqDWAy
- Ippolito E, Natali PG, Postacchini F, Accinni L, De Martino C (1977) Ultrastructural and immunochemical evidence of actin in the tendon cells. Clin Orthop 126: 282-284. Link: https://bit.ly/2WIavMi
- Díaz-Flores L, Gutiérrez R, Díaz-Flores L, Goméz MG, Sáez FJ, et al. (2016) Behaviour of telocytes during physiopathological activation. Semin Cell Dev Biol 55: 50-61. Link: https://bit.ly/3mQm4LK
- Díaz-Flores L, Gutiérrez R, García MP, Sáez FJ, Díaz-Flores L et al. (2014) CD34+ stromal cells/fibroblasts/fibrocytes/telocytes as a tissue reserve and a principal source of mesenchymal cells. Location, morphology, function and role in pathology. Histol Histopathol 29: 831-870. Link: https://bit.ly/37LGYrf
- Wipff PJ, Hinz B (2009) Myofibroblasts work best under stress. J Bodyw Mov Ther 13: 121-127. Link: https://bit.ly/2WMZd9q
- Weiler A, Unterhauser FN, Bail HJ, Huning M, Haas NP (2002) Alpha-smooth muscle actin is expressed by fibroblastic cells of the ovine anterior cruciate ligament and its free tendon graft during remodeling. J Orthop Res 20: 310-317. Link: https://bit.ly/3aHUk9T
- O'Shea K, Wolfe SW (2013) Two-stage reconstruction with the modified Paneva-Holevich technique . Hand Clin 29: 223-233. Link: https://bit.ly/3mOPni1
- Rooney SI, Torino DJ, Baskin R, Vafa RP, Kuntz AF, et al. (2017) Rat supraspinatus tendon responds acutely and chronically to exercise. J Appl Physiol 123: 757-763. Link: https://bit.ly/38Qqn59 .
- Rooney SI, Baskin R, Torino DJ, Vafa RP, Khandekar PS, et al. (2016) Ibuprofen Differentially Affects Supraspinatus Muscle and Tendon Adaptations to Exercise in a Rat Model. Am J Sports Med 44: 2237-2245. Link: https://bit.ly/3hh8JeB
- Duci SB, Arifi HM, Ahmeti HR, Manxhuka-Kerliu S, Neziri B, et al. (2015) Biomechanical and Macroscopic Evaluations of the Effects of 5-Fluorouracil on Partially Divided Flexor Tendon Injuries in Rabbits . Chin Med J (Engl) 128: 1655-1661. Link: https://bit.ly/34Mmsov
- Xia CS, Hong GX, Dou RR, Yang XY (2005) Effects of CTN on cell proliferation and collagen pro-duction of tendon sheath fibroblasts, epitenon tenocytes, and endotenon tenocytes. Chin J Traumatol 8: 369-374. Link: https://bit.ly/34HvlzS
- Wu YF, Mao WF, Zhou YL, Wang XT, Liu PY, et al. (2016) Adeno-associated virus-2-mediated TGF-β1 microRNA transfection inhibits adhesion formation after digital flexor tendon injury. Gene Ther 23: 167-175. Link: https://bit.ly/37P6D2j
- Bonifasi-Lista C, Lake SP, Small MS, Weiss JA (2005) Viscoelastic properties of the human medial collateral ligament under longitudinal, transverse and shear loading. J Orthop Res 23: 67-76. Link: https://bit.ly/3rtW50j
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
