International Journal of Oral and Craniofacial Science
Preparation of Nano-drug Carrier by Microfluidic Technology for Oral Cancer Treatment
School of Transportation, Shandong Province Key Laboratory of High Performance Hard Alloys and Precision Tools, Ludong University, China
Author and article information
Cite this as
Tang T, Liu Y, Chen X, Chen X. Preparation of Nano-drug Carrier by Microfluidic Technology for Oral Cancer Treatment. Int J Oral Craniofac Sci. 2025;11(1):001-003. Available from: 10.17352/ijocs.000065
Copyright License
© 2025 Tang T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Abstract
In oral cancer therapy, traditional chemotherapeutic drugs cannot meet clinical needs due to poor targeting, low bioavailability, and severe side effects. Microfluidic technology, with precise fluid manipulation, enables efficient preparation of nanoparticulate drug carriers, realizing “precision fabrication” and “functional design” of nanocarriers. By adapting to oral cancer’s pathological characteristics and personalized treatment needs, it provides a new pathway for oral cancer therapy breakthroughs, with its technical advantages and application potential gradually emerging.
Introduction
Traditional nanocarriers (e.g., liposomes, polymeric nanoparticles) are prepared via emulsion-solvent evaporation or sonication, suffering from broad particle size distribution (PDI often > 0.2), large batch-to-batch variations, and unstable drug loading—severely reducing clinical translation value. Microfluidic technology solves these bottlenecks by precisely controlling mass transfer and mixing, as validated in multiple studies.
Methodology
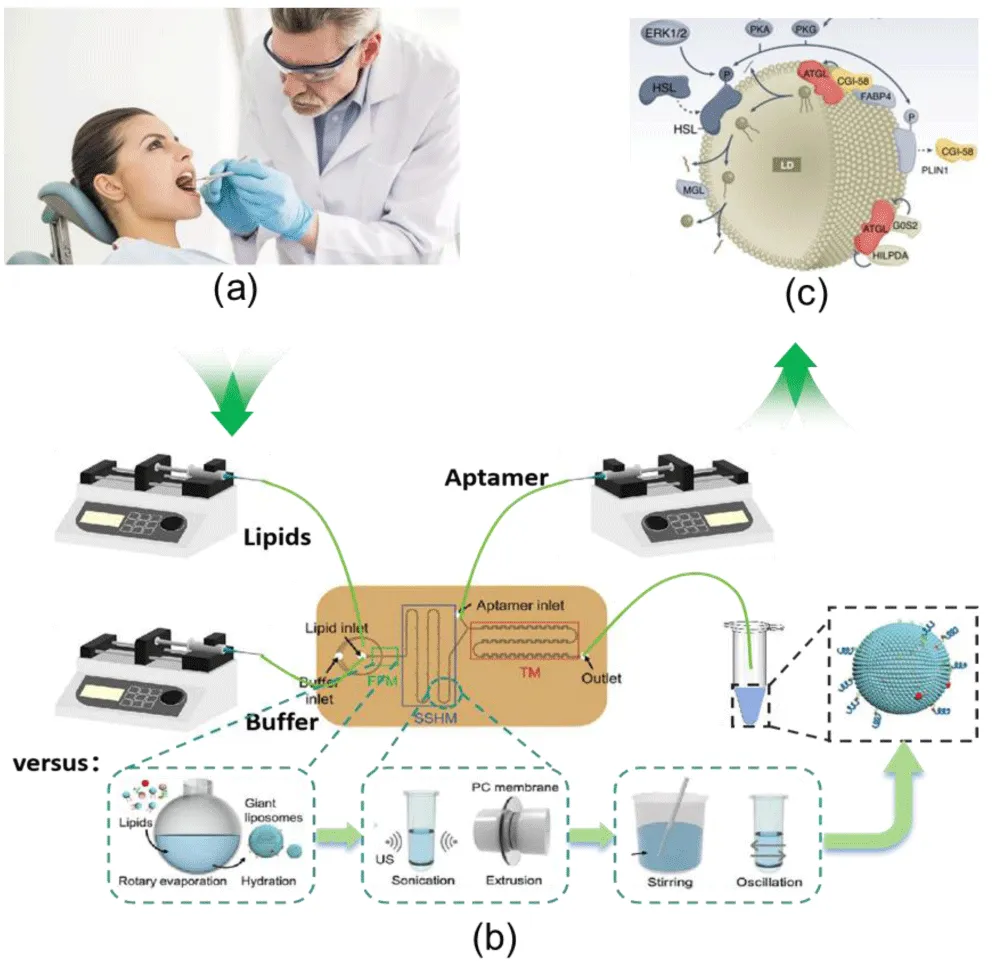
Researchers used a microfluidic swirl mixer to prepare curcumin (Cur)-loaded gelatin nanoparticles (GNPs). By optimizing gelatin concentration, pH, two-phase flow rate ratio (FRR) and total flow rate (TFR), they obtained uniform carriers with average particle size 90 ± 7 nm (PDI < 0.1). Compared with traditional emulsification (particle size 150–300 nm, PDI > 0.15), microfluidic carriers are more conducive to penetrating oral cancer tumor interstitium (nanoparticles < 200 nm accumulate via EPR effect), with batch-to-batch drug loading variation only 3.2% (vs. 12.5% for traditional methods). This device achieves 320 mL/min flow rate, 0.1 ms rapid mixing, and milligram-scale single-batch preparation, overcoming traditional microfluidics’ “low volume, hard scaling” issue and highlighting industrial potential [1,2]. Innovatively combining 3D printing with microfluidics, researchers designed two T-shaped microchannel devices (spiral mixing channel for Device 1, Fishbone-structured for Device 2). Series connection plus ultrasound enabled efficient fusion of glioma cell membranes with lipids for biomimetic liposomes. The core logic of this technology — precisely controlling the fusion ratio of cell membranes and lipids via microfluidics to achieve “homotypic targeting” — has been initially verified using glioma models: the 3D-printed microfluidic device achieved uniform particle size (PDI < 0.1) of glioma-targeted liposomes and high membrane fusion efficiency (fusion rate > 70% verified by FRET), confirming the feasibility of the microfluidic process for preparing biomimetic nanocarriers” This logic applies directly to oral cancer: oral cancer cells (e.g., CAL-27, SCC-9) have unique surface antigens (EGFR, CD44). Using the same 3D-printed device to fuse oral cancer cell membranes with lipids prepares “homotypic targeting” nanocarriers, which bypass oral mucosal barriers and immune clearance via “self-recognition” to enhance tumor accumulation. 3D-printed devices also allow “customization” (adjusting channel size and mixing mode for subtypes like tongue cancer or buccal mucosal cancer), matching oral cancer’s heterogeneity. Microfluidic nanocarriers address core oral cancer therapy challenges via multi-responsive design, targeted modification, and theranostics integration [3-5]. Oral squamous cell carcinoma’s tumor microenvironment (acidity: pH 5.5–6.5, hypoxia, NIR permeability) allows microfluidic carriers to respond to signals for controlled drug release. Researchers loaded CuS NPs and Fe₃O₄ NPs into GNPs: CuS converts 980 nm NIR light to heat (photothermal therapy) and triggers gelatin relaxation to accelerate Cur release; Fe₃O₄ enhances targeted accumulation via external magnetic field and regulates release via magnetothermal effect. In vitro tests showed: at pH 5.5 (simulated tumor environment), 50% Cur released in 40 h (vs. 58 h at pH 7.4, normal tissue); NIR irradiation increased release rate by 2.3 times, and MCF-7 cell apoptosis (The targeting and cytotoxicity of human breast cancer MCF-7 cells were verified. This cell line highly expresses folate receptor (FR), with a receptor expression level of approximately 1.2 × 10⁵ per cell, which is highly consistent with the expression characteristics of FR in various subtypes of oral cancer cells, such as tongue cancer CAL-27 cells and buccal mucosa cancer SCC-9 cells. This paper compared the cellular uptake efficiency and apoptosis rate of MCF-7 (high FR) and A549 (human lung cancer cells, low FR), confirming the targeting enrichment ability of folic acid-modified gelatin nanoparticles (Fe₃O₄/CuS/Cur@GNPs-FA) for FR-positive cells - the uptake efficiency of MCF-7 cells for this carrier was 4.2 times higher than that of A549, and the apoptosis rate reached 95% after NIR irradiation (significantly higher than 42% of free curcumin). This result can be analogously supported that this folic acid targeting strategy is also applicable to FR-positive oral cancer cells, providing a technical basis for subsequent specific model verification of oral cancer [6-8] (Figure 1).
Targeted modification solves carrier penetration, enzyme degradation, and tumor recognition issues. In the GNPs study, “one-step modification” during microfluidics conjugated folic acid (FA) to carriers: FA-modified carriers had 19.4x higher uptake by folate receptor-high oral cancer cells (e.g., tongue cancer cells) at 2 h. In a mixed cell model (A549 as normal cells, MCF-7 as tumor cells), carrier accumulation in MCF-7 reached 82%, reducing normal cell toxicity. Homotypic targeting (oral cancer cell membrane modification) further improves precision: carriers evade macrophage clearance and bind tumor cells via E-cadherin, aiding in treating metastases.
Combined with liquid biopsy (saliva CTC/ctDNA detection), microfluidics forms a “diagnosis-carrier preparation-therapy” closed loop: microfluidic chips screen patient-specific markers (EGFR mutations, HPV DNA), and adjust carrier targeting ligands (EGFR antibodies, HPV capsid proteins). For example, HPV-targeted carriers improve recognition of HPV-positive oropharyngeal cancer cells, matching oral cancer’s etiological heterogeneity. Theranostics integration addresses efficacy monitoring and recurrence warning. Microfluidics plus nanodiagnostics builds “detection-therapy” systems: e.g., quantum dots (QDs) and drugs co-loaded in carriers—QDs monitor distribution and release via fluorescence imaging, carriers deliver drugs. “Microfluidic-nanosensor” systems detect post-treatment saliva markers (CYFRA 21-1, IL-8) and adjust drug dosage using fluorescent signals, solving delayed efficacy evaluation and early recurrence detection issues [9].
Most studies use glioma/breast cancer models; little research focuses on mucosal penetration and salivary enzyme stability. Subtypes (tongue cancer, oropharyngeal cancer) have distinct pathology—”universal” carriers fail to meet needs. Most studies are lab-scale (<100 mg/batch), but clinical use requires gram-kilogram scale. Parallel device connection and channel optimization are needed. Most efficacy tests are in vitro; oral cancer animal models (hamster cheek pouch cancer, nude mouse tongue cancer) and clinical sample validation (tumor tissues, saliva) are absent, limiting evaluation of in vivo efficacy and toxicity. Mechanisms for guiding carrier preparation via diagnostic results and standardized “diagnosis-preparation-therapy” procedures are lacking [10].
Future development should focus on Add mucosal penetration peptides (e.g., TAT) to enhance mucosal crossing; develop HPV L1-modified carriers for HPV-positive oropharyngeal cancer; design degradable carriers (gelatin, chitosan) to prevent mucosal irritation. Achieve gram-scale preparation via parallel channels and continuous flow optimization; set up quality control (particle size/PDI/drug loading variation <5%); verify carrier accumulation, release, and safety in animal models; run small-scale clinical trials (advanced oral cancer palliative treatment). Connect diagnostic chips (saliva CTC/ctDNA detection) with carrier preparation chips to adjust ligands, drug loading, and response mechanisms in real time; use carrier fluorescence imaging to monitor efficacy and form a closed-loop system.
Conclusion
Microfluidic technology, integrated with nanomaterials, targeting, and diagnostics, reshapes oral cancer therapy: it solves traditional carrier uniformity via precision fabrication, adapts to therapy needs via functional design, and enables personalization via theranostics. Despite current limitations, with deeper “technology-disease-clinic” integration, microfluidic nanocarriers will drive oral cancer therapy from “broad-spectrum chemotherapy” to “precision therapy,” improving patient prognosis.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author Contributions
Tao Tang was responsible for literature review, data collation and analysis, and drafting the initial manuscript.
Yuchen Liu and Xinkun Chen handled figure and table preparation, literature collation, and data verification.
Xueye Chen provided funding support, and was responsible for revising and finalizing the manuscript.
References
- Xia Y, Xu R, Ye S, Yan J, Kumar P, Zhang P, et al. Microfluidic formulation of curcumin-loaded multiresponsive gelatin nanoparticles for anticancer therapy. ACS Biomater Sci Eng. 2023;9:3402–3413. Available from: https://doi.org/10.1021/acsbiomaterials.3c00318
- Pillai S, Kwan JC, Yaziji F, Yu H, Tran SD. Mapping the potential of microfluidics in early diagnosis and personalized treatment of head and neck cancers. Cancers (Basel). 2023;15(15):3894. Available from: https://doi.org/10.3390/cancers15153894
- Arduino I, Di Fonte R, Sommonte F, Lopedota A, Porcelli L, Li J, et al. Fabrication of biomimetic hybrid liposomes via microfluidic technology: homotypic targeting and antitumor efficacy studies in glioma cells. Int J Nanomedicine. 2024;19:13217–13233. Available from: https://doi.org/10.2147/IJN.S489872
- Senevirathna K, Jayawickrama SM, Jayasinghe YA, Prabani KIP, Akshala K, Pradeep RGGR, et al. Nanoplatforms: the future of oral cancer treatment. Health Sci Rep. 2023;6:e1471. Available from: https://doi.org/10.1002/hsr2.1471
- Liu Y, Yang G, Hui Y, Ranaweera S, Zhao CX. Microfluidic nanoparticles for drug delivery. Small. 2022;18(36):e2106580. Available from: https://doi.org/10.1002/smll.202106580
- Zoupanou S, Volpe A, Primiceri E, Gaudiuso C, Ancona A, Ferrara F, et al. SMILE platform: an innovative microfluidic approach for on-chip sample manipulation and analysis in oral cancer diagnosis. Micromachines. 2021;12:885. Available from: https://www.mdpi.com/2072-666X/12/8/885
- Chen X, Pan Y, Tang T, Fu J, Chen X, Bao C. Machine learning-guided one-step fabrication of targeted emodin liposomes via novel micromixer for ulcerative colitis therapy. Nano Res. 2025;18:94907713. Available from: https://doi.org/10.26599/NR.2025.94907713
- Ghasemi Toudeshkchouei M, Zahedi P, Shavandi A. Microfluidic-assisted preparation of 5-fluorouracil-loaded PLGA nanoparticles as a potential system for colorectal cancer therapy. Materials (Basel). 2020;13(7):1483. Available from: https://doi.org/10.3390/ma13071483
- Ejeta F. Recent advances of microfluidic platforms for controlled drug delivery in nanomedicine. Drug Des Devel Ther. 2021;15:3881–3891. Available from: https://www.tandfonline.com/action/showCitFormats?doi=10.2147/DDDT.S324580
- Xu L, Wang X, Liu Y, Yang G, Falconer RJ, Zhao CX. Lipid nanoparticles for drug delivery. Adv NanoBiomed Res. 2022;2:2100109. Available from: https://doi.org/10.1002/anbr.202100109
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
