International Journal of Oral and Craniofacial Science
Transosteal radial free flap in palate reconstruction
Luis Nieto1*, Oscar De León2, Rolf Smit3, Zamir Paéz4 and Camilo Cháves5
2Maxillofacial Surgery, Pontifical Javeriana University, Colombia
3Plastic and Microsurgeon, Barranquilla, Colombia
4Plastic Surgeon, Medellín, Colombia
5Resident of Plastic Surgery, Pontifical Javeriana University, Colombia
Cite this as
Nieto L, León OD, Smit R, Paéz Z, Cháves C (2022) Transosteal radial free flap in palate reconstruction. Int J Oral Craniofac Sci 8(2): 032-035. DOI: 10.17352/2455-4634.000057Copyright License
© 2022 Nieto L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The reconstruction of the palate has been a challenge for the reconstructive surgeon, due to the multiple complications that arise, such as infection, dehiscence, and fall of the flap used. We present the description of a new radial free flap fixation technique, commonly used for this type of reconstruction. This transosteal fixation technique prevents dehiscence and flap descent in all cases performed, by combining two widely used procedures, the radial free flap, and the Lefort I osteotomy, with excellent results.
Introduction
The palate comprises bone, and muscular parts, suspended between the oropharynx and the nasopharynx. The hard palate formed by bone originates from a fusion in the midline of the palatal processes of the maxilla and the horizontal processes of the palatine bone. The mobile, muscular part is the soft palate wrapped in mucous membranes. In their entirety, they make up a supporting structure for the middle third of the face which separates the oral cavity, the antral, and the orbital cavity, being a support for the eyeballs, lower eyelids, cheeks, lips and nose. It plays an important role in maintaining the physiology of speech, swallowing, and chewing [1].
Most acquired defects in the palate correspond to secondary defects because of oncological resections of the head and neck [2]. Inadequate management of these defects leads to solid and liquid food leaks into the nasal cavity, swallowing dysfunction, alterations in chewing, hypernasality, velopharyngeal incompetence, and loss of support for the soft tissues of the middle third of the face [3]. Surgical procedures and prostheses can be used alone or together, for their reconstruction and rehabilitation, depending on the size, location of the defect in the palate, and the basic medical condition of each patient [4].
Therefore, it is necessary to create new surgical techniques or a combination of existing ones, to optimize the results of the reconstruction of palatal defects, especially those defects considered large and that affect swallowing and language functions. This work is an effort to achieve this goal.
Background
The objectives of reconstruction are to separate the oral and sinunasal cavities, reestablish adequate nasal breathing, and normalize chewing, swallowing, and speech [5]. Prosthetic restoration with obturators was for a long time considered the best method for the “reconstruction” of defects in the hard palate, separating the oral cavity, maintaining a seal against the mucosa, and allowing intelligible language and better swallowing [6].
The size of the palatal defect to be reconstructed defines the method to be used and, at the same time, is the most important factor for the presentation of postoperative complications that may arise, in addition to determining the degree of functional impairment for each patient.
Large defects are associated with a poor functional prognosis with prostheses [7], where the soft tissue available for prosthesis support decreases, with persisting speech disturbances, regurgitation of solids and liquids towards the nasal cavity, and difficulty maintaining the hygiene of the cavity generated by maxillectomy, and the need to make repeated adjustments to the prostheses because of changes in size and shape of the defect in the palate [8].
Multiple surgical procedures have been described for palate reconstruction, palatal mucosa flaps, flaps from oral cavity tissues, such as the buccinator muscle flap [9], flaps based on facial vessels [10], pharyngeal flaps [11] and uvulopalatal flaps, among others, with inconsistent results, because of the limited availability of tissues and the permanent contamination from the exposed nasal cavity and maxillary sinus [12].
Microsurgical techniques have made it possible to transfer the tissues with complete defect obliteration, achieving adequate functional reconstructions in most cases [13]. Moreno, et al, propose reconstruction with free microvascular flaps for defects greater than 50% of the palate, or anterior palate defects with canine involvement [14]. Genden, et al. demonstrated that reconstruction with free flaps improves the quality of life, even in patients with defects less than 50% [15].
Free, scapular tip [16], latissimus dorsi, and anterolateral thigh (ALT) [17,18] flaps have been described, obtaining good postoperative results [19]. The radial free flap has been widely used in the reconstruction of soft palate defects, because of its pliability, thinness, and ability to allow multiple forms [20-22]. Roh, et al, have used this flap together with the palmaris longus muscle tendon to perform a functional reconstruction by repairing the levator band mechanism [23]. H. Lim. et al, concluded that the radial free flap is especially suitable in this type of reconstruction, thanks to its thinness, flexibility, and the ability to maintain a constant volume and surface area over time, allowing adequate mobility of the oral and oropharyngeal structures rebuilt [24].
Jeong, et al. report frequent complications when performing this flap, with distortion and dehiscence, because of its weight, continuous tongue movements, the force of gravity, and bacterial contamination from the sinonasal cavities [25]. To avoid these complications, the obturator as a splint has been used, acting as a support, and avoiding flap descent, as well as slit-shaped fenestrations at the level of the midline of the hard palate [26] and use of the Palmaris longus tendon, included in the radial flap, as a suspension [27,28].
Materials and methods
We define palate defects into 3 groups based on the classification proposxed by Roh, et al. [23]. Group I defect involved up to one-fourth of the soft palate and/or the ipsilateral lateral pharyngeal wall. Group II defect involved up to one-half of the soft palate whether including the uvula. Group III defect involved more than three-fourths of the soft palate and always included the uvula and/or lateral pharyngeal wall.
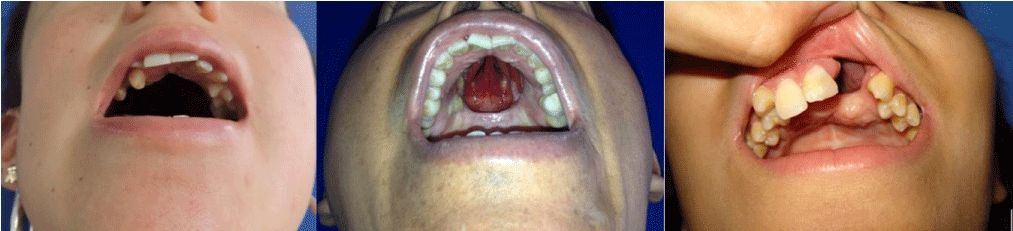
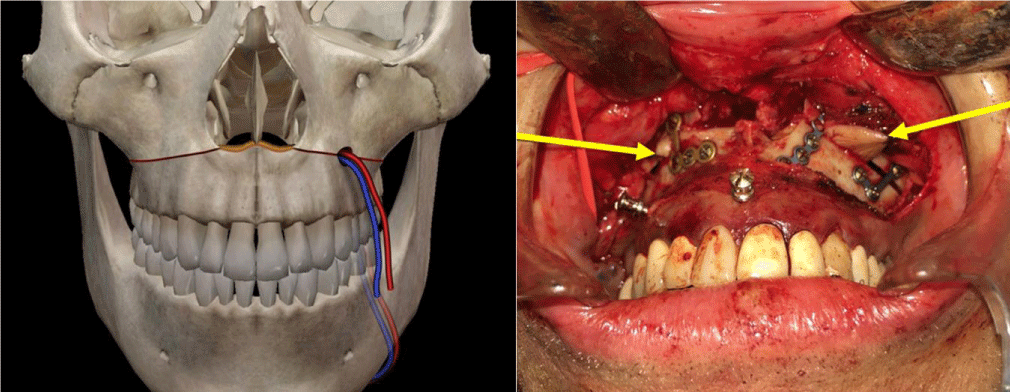
To date, this surgical technique has been performed in 6 patients with palatal defects classified in Groups II and III, (Table 1) (Figure 1). All patients and their relatives were sufficiently informed of the nature of the surgical intervention, surgical techniques widely used by the senior authors, the possible results, and complications, and signing the corresponding informed consent.
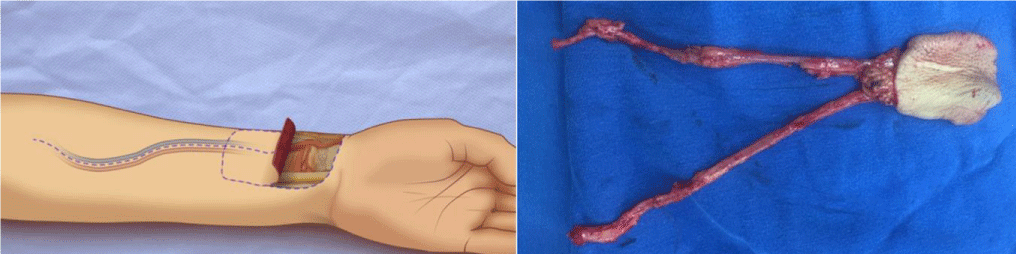
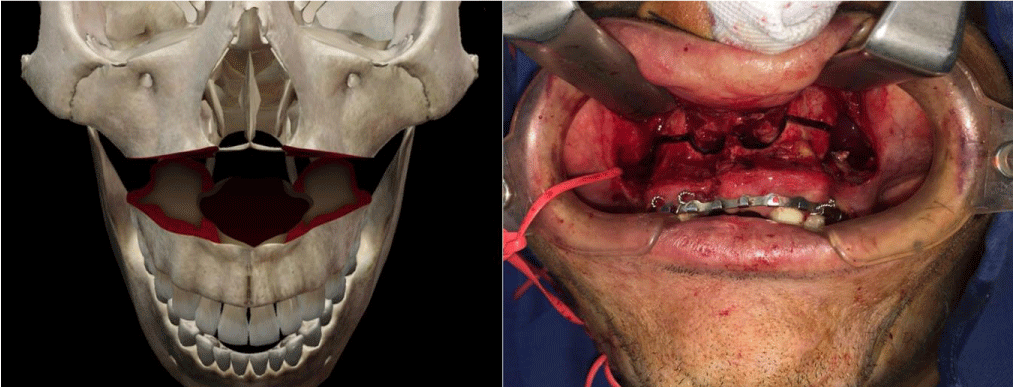
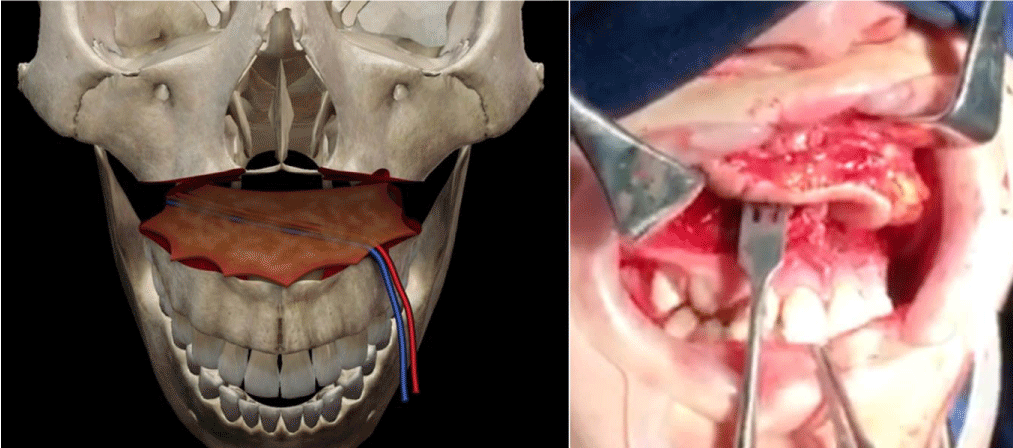
The technique is the combination of two known surgical procedures described many years ago for different indications, Lefort I osteotomy and free radial flap (Figure 2). The first step is the realization of a conventional Lefort I osteotomy of the maxilla (Figure 3), separating the two bone segments enough to then interpose the flap. At the same time with other surgical teams or in a second step, conventional harvesting of the radial free flap with dimensions according to the size of the maxilla and length of pedicle at least 12 cm, so as not to have difficulty when performing the microvascular anastomosis in the recipient vessels, which for all our patients was performed in the facial vessels. The third step, is de-epithelialization of the skin island, except for the segment that corresponds to the dimensions of the palatal defect to be reconstructed. Fourth step, pedicle division in the antebrachial area, transposition of the flap and placement between the two portions of the maxilla (Figure 4), leaving the edges of the flap “trapped” between the bone segments, making their replacement (Figure 5) and fixation with osteosynthesis material, taking care that the pedicle is not compressed, thus allowing its stable and fixed suspension. The fifth step is microvascular anastomoses to chosen receptor vessels and closed incisions. It is unnecessary to leave intermaxillary fixation on, a soft diet for at least 4 to 6 weeks until adequate bone healing has been achieved.
Results
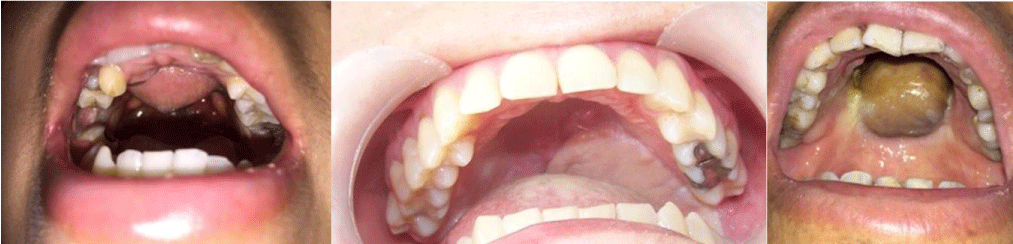
With at least 1 year follow-up, all patients got satisfactory results in terms of flap survival, adequate swallowing, and intelligible language (Figure 6). No patient presented flap descent that interfered with chewing, oral breathing, or swallowing. In one patient there was small anterior dehiscence that was easily solved, with repositioning of the flap. In all cases was obtained adequate healing to the adjacent tissues, and avoiding the possibility of shedding, is the main complication in this kind of reconstruction.
Discussion
The successful reconstruction of defects at the hard and soft palate remains a reconstructive challenge, which depends on the size and extent of the defect [29]. For most authors, it requires a functional reconstruction of the palate for normal speech and swallowing. This reconstruction must consider the need for bone or soft tissue support and separation of the nasal and oral cavities.
Multiple methods have been described for the closure of defects after oncological resections, such as the latissimus dorsi, rectus abdominis, and the radial free flap, with or without bone reconstruction. The prosthetic filling has long been considered the method of choice for small to medium defects, but microsurgical reconstruction has shown superior speech and swallowing results for extensive or anterior defects. As mentioned above, microsurgical reconstruction has been shown to improve the quality of life, unlike prosthetic rehabilitation, even in patients with small and medium defects [30].
The radial flap is a thin, moldable flap that is suitable for oral and oropharyngeal defects. It can keep a consistent volume and surface over time. Although it may be a favorite method of choice, a successful reconstruction can be disturbed by a lowering of the flap from the position because of a lack of fixation support [31].
In this article, we propose a new surgical technique for free radial flap suspension by means of train steal fixation using a Le Fort I osteotomy. It protects the free radial flap from tongue mobility and reduces contact with salivary production. It achieves a greater seal of the oronasal fistula created by the oncological resection, thus allowing restoration of phonatory and swallowing functions.
Adequate experience is required in performing maxillary osteotomies and microsurgical free flaps, to achieve a successful combination with good results.
We think that the main indication for this surgical technique is defects of more than 50% of the palate, without maxillary defects. Contraindications would be in patients with comorbidities that limit the performance of free flaps and previous maxillary surgeries.
Conclusion
The size and extent of the palate defect palate are the best predictors of speech and swallowing. Prosthetic rehabilitation is a choice for small and medium palate defects, yet microsurgical reconstruction provides better speech and swallowing, allowing better support and long-lasting outcomes. This approach is repeatable in the same way or with different flaps.
This article is limited by the small number of patients, although excellent results were obtained in all cases, which is why we consider it to be a good alternative for reconstruction in this type of defect. We need further studies with more patients undergoing this procedure with longer-term follow-ups to offer better and more consistent conclusions of this new proposal.
- Gupta V, Cohan DM, Arshad H, Kuriakose MA, Hicks WL Jr. Palatal reconstruction. Curr Opin Otolaryngol Head Neck Surg. 2012; Aug;20(4):225-30. doi: 10.1097/MOO.0b013e328355389f. PMID: 22894989.
- Dalgorf D, Higgins K. Reconstruction of the midface and maxilla. Curr Opin Otolaryngol Head Neck Surg. 2008 Aug;16(4):303-11. doi: 10.1097/MOO.0b013e328304b426. PMID: 18626247.
- Chang EI, Hanasono MM. State-of-the-art reconstruction of midface and facial deformities. J Surg Oncol. 2016 Jun;113(8):962-70. doi: 10.1002/jso.24150. PMID: 27226161.
- van der Sloot PG. Hard and soft palate reconstruction. Curr Opin Otolaryngol Head Neck Surg. 2003 Aug;11(4):225-9. doi: 10.1097/00020840-200308000-00001. PMID: 14515067.
- Nuri T, Ueda K, Yamada A, Okada M, Hara M. Reconstruction of the dynamic velopharyngeal function by combined radial forearm-palmaris longus tenocutaneous free flap, and superiorly based pharyngeal flap in postoncologic total palatal defect. Ann Plast Surg. 2015 Apr;74(4):437-41. doi: 10.1097/SAP.0b013e3182a63618. PMID: 25749212.
- Okay DJ, Genden E, Buchbinder D, Urken M. Prosthodontic guidelines for surgical reconstruction of the maxilla: a classification system of defects. J Prosthet Dent. 2001 Oct;86(4):352-63. doi: 10.1067/mpr.2001.119524. PMID: 11677528.
- Rieger J, Bohle Iii G, Huryn J, Tang JL, Harris J, Seikaly H. Surgical reconstruction versus prosthetic obturation of extensive soft palate defects: a comparison of speech outcomes. Int J Prosthodont. 2009 Nov-Dec;22(6):566-72. PMID: 19918590.
- Sharif KF, Sims JR, Yue LE, Baik FM, Kiplagat KJ, Buchbinder D, Okay DJ, Chai RL, Urken ML. Reconstructive and prosthodontic outcomes after multiple palatomaxillary reconstructions. Laryngoscope. 2020 Oct;130(10):2349-2353. doi: 10.1002/lary.28481. Epub 2019 Dec 30. PMID: 31886884.
- Massarelli O, Vaira LA, Gobbi R, Biglio A, Dell'aversana Orabona G, De Riu G. Soft palate functional reconstruction with buccinator myomucosal island flaps. Int J Oral Maxillofac Surg. 2018 Mar;47(3):316-323. doi: 10.1016/j.ijom.2017.11.012. Epub 2017 Dec 8. PMID: 29225008.
- Albert S, Carmantrant R, Panajotopoulos A, Charrier JB, Barry B. Utilisation du lambeau myomuqueux pédiculé par l'artère faciale dans les reconstructions palatines [Reconstruction of hard palate defects using a facial artery musculomucosal flap]. Ann Chir Plast Esthet. 2008 Jun;53(3):281-4. French. doi: 10.1016/j.anplas.2007.02.012. Epub 2007 Apr 3. PMID: 17408836.
- Karle WE, Anand SM, Clain JB, Scherl S, Urken ML. Total soft palate reconstruction using the palatal island and lateral pharyngeal wall flaps. Laryngoscope. 2013 Apr;123(4):929-33. doi: 10.1002/lary.23787. Epub 2012 Nov 20. PMID: 23169602.
- Seikaly H, Rieger J, Zalmanowitz J, Tang JL, Alkahtani K, Ansari K, O'Connell D, Moysa G, Harris J. Functional soft palate reconstruction: a comprehensive surgical approach. Head Neck. 2008 Dec;30(12):1615-23. doi: 10.1002/hed.20919. PMID: 18798302.
- Costa H, Zenha H, Sequeira H, Coelho G, Gomes N, Pinto C, Martins J, Santos D, Andresen C. Microsurgical reconstruction of the maxilla: Algorithm and concepts. J Plast Reconstr Aesthet Surg. 2015 May;68(5):e89-e104. doi: 10.1016/j.bjps.2014.12.002. Epub 2015 Jan 14. PMID: 25778873.
- Moreno MA, Skoracki RJ, Hanna EY, Hanasono MM. Microvascular free flap reconstruction versus palatal obturation for maxillectomy defects. Head Neck. 2010 Jul;32(7):860-8. doi: 10.1002/hed.21264. PMID: 19902543.
- Genden EM, Wallace DI, Okay D, Urken ML. Reconstruction of the hard palate using the radial forearm free flap: indications and outcomes. Head Neck. 2004 Sep;26(9):808-14. doi: 10.1002/hed.20026. PMID: 15350027.
- Pagedar NA, Gilbert RW, Chan H, Daly MJ, Irish JC, Siewerdsen JH. Maxillary reconstruction using the scapular tip free flap: a radiologic comparison of 3D morphology. Head Neck. 2012 Oct;34(10):1377-82. doi: 10.1002/hed.21946. Epub 2012 Jan 27. PMID: 22287228.
- Ozkan O, Ozkan O, Coskunfirat OK, Hadimioğlu N. Reconstruction of large palatal defects using the free anterolateral thigh flap. Ann Plast Surg. 2011 Jun;66(6):618-22. doi: 10.1097/SAP.0b013e3181e35cd8. PMID: 21178759.
- Zenga J, Sharon JD, Gross J, Gantz J, Pipkorn P. Soft palate reconstruction after radionecrosis: Combined anterolateral thigh adipofascial and nasoseptal flaps. Auris Nasus Larynx. 2018 Aug;45(4):875-879. doi: 10.1016/j.anl.2017.11.003. Epub 2017 Nov 13. PMID: 29146179.
- Herzog M, Grafmans D, Plontke SK, Bartel S, Plößl S. Funktionelle Ergebnisse nach Rekonstruktion des weichen Gaumens bei Patienten mit Oropharynxkarzinom [Functional results after soft palate reconstruction in oropharyngeal cancer patients]. HNO. 2021 Feb;69(2):122-130. German. doi: 10.1007/s00106-020-00839-8. PMID: 32128602.
- Kim JH, Chu HR, Kang JM, Bae WJ, Oh SJ, Rho YS, Ahn HY, Jung CH. Functional benefit after modification of radial forearm free flap for soft palate reconstruction. Clin Exp Otorhinolaryngol. 2008 Sep;1(3):161-5. doi: 10.3342/ceo.2008.1.3.161. Epub 2008 Sep 30. PMID: 19434250; PMCID: PMC2671750.
- Duflo S, Lief F, Paris J, Giovanni A, Thibeault S, Zanaret M. Microvascular radial forearm fasciocutaneous free flap in hard palate reconstruction. Eur J Surg Oncol. 2005 Sep;31(7):784-91. doi: 10.1016/j.ejso.2005.05.008. PMID: 16002257.
- Moubayed SP, Osorio M, Buchbinder D, Lazarus C, Urken ML. Soft palate reconstruction using a combination of a turn-in flap and a radial forearm flap. Laryngoscope. 2017 Aug;127(8):1772-1774. doi: 10.1002/lary.26462. Epub 2017 Jan 4. PMID: 28052425.
- Roh TS, Lee WJ, Choi EC, Koh YW, Lew DH. Radial forearm-palmaris longus tenocutaneous free flap; implication in the repair of the moderate-sized postoncologic soft palate defect. Head Neck. 2009 Sep;31(9):1220-7. doi: 10.1002/hed.21093. PMID: 19360739.
- Kang HG, Park MC, Lim H, Kim JH, Lee IJ. Modified folding radial forearm flap in soft palate and tonsillar fossa reconstruction. J Craniofac Surg. 2013 Mar;24(2):458-60. doi: 10.1097/SCS.0b013e31826cfecf. PMID: 23524714.
- Jeong EC, Jung YH, Shin JY. Use of Postoperative Palatal Obturator After Total Palatal Reconstruction With Radial Forearm Fasciocutaneous Free Flap. J Craniofac Surg. 2015 Jul;26(5):e383-5. doi: 10.1097/SCS.0000000000001856. PMID: 26114541.
- Sakuraba M, Kimata Y, Ota Y, Uchiyama K, Kishimoto S, Harii K, Ebihara S. Simple maxillary reconstruction using free tissue transfer and prostheses. Plast Reconstr Surg. 2003 Feb;111(2):594-8; discussion 599-600. doi: 10.1097/01.PRS.0000041941.98504.B6. PMID: 12560680.
- Lee MC, Lee DW, Rah DK, Lee WJ. Reconstruction of a total soft palatal defect using a folded radial forearm free flap and palmaris longus tendon sling. Arch Plast Surg. 2012 Jan;39(1):25-30. doi: 10.5999/aps.2012.39.1.25. Epub 2012 Jan 15. PMID: 22783487; PMCID: PMC3385305.
- Pang KP, Vicini C, Montevecchi F, Piccin O, Chandra S, Yang HC, Agrawal V, Chung JCK, Chan YH, Pang SB, Pang KA, Pang EB, Rotenberg B. Long-term Complications of Palate Surgery: A Multicenter Study of 217 Patients. Laryngoscope. 2020 Sep;130(9):2281-2284. doi: 10.1002/lary.28432. Epub 2019 Nov 25. PMID: 31765026.
- Sinha UK, Young P, Hurvitz K, Crockett DM. Functional outcomes following palatal reconstruction with a folded radial forearm free flap. Ear Nose Throat J. 2004 Jan;83(1):45-8. PMID: 14986758.
- Larson DL. A Classification System and Algorithm for Reconstruction of Maxillectomy and Midfacial Defects. Plast Reconstr Surg. 2000 Jun;105(7):2347-2348. doi: 10.1097/00006534-200006000-00005. PMID: 11242346.
- Jategaonkar AA, Kaul VF, Lee E, Genden EM. Surgery of the Palatomaxillary Structure. Semin Plast Surg. 2020 May;34(2):71-76. doi: 10.1055/s-0040-1709430. Epub 2020 May 6. PMID: 32390773; PMCID: PMC7202911.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
