Journal of Dental Problems and Solutions
Involvement of trigeminal neuralgia in type 2 diabetes
Tomislav Badel1*, Miroslav Hrelja2, Jelena Bošnjak3, Dijana Zadravec4, Matea Prenc4 and Mia Smoljan4
2Dental office, Dugo Selo, Croatia
3University of Zagreb, Faculty of Chemical Engineering and Technology, Marulićev trg 19, HR-10000, Zagreb, Croatia
4Department of Diagnostic and Interventional Radiology, Clinical Hospital Centre “Sisters of Mercy”, The University of Zagreb, Zagreb, Croatia
Cite this as
Badel T, Hrelja M, Bošnjak J, Zadravec D, Prenc M, et al. (2023) Involvement of trigeminal neuralgia in type 2 diabetes. J Dent Probl Solut 10(2): 010-015. DOI: 10.17352/2394-8418.000122Copyright License
© 2023 Badel T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The aim of this paper is to describe orofacial neuralgic pain related to diabetes mellitus pathology with a clinical report of a female patient who suffered from diabetic polyneuropathy. A 61-year-old female patient was treated neurologically and dentally due to suspicions of Trigeminal Neuralgia (TN) and disorders of the temporomandibular joint. Recent symptoms were burning and heat, electric shock sensation related to the right side of the face and particularly the second right premolar tooth. The patient had received regular insulin therapy (type 2 diabetes mellitus) for the last 10 years and was diagnosed with diabetic polyneuropathy with a higher value of glycosylated hemoglobin HbA1c (59 mmol/mol). The psychological evaluation showed an elevated anxiety level according to Spielberger’s State-Trait Anxiety Inventory. The most common neurogenic complication of type 2 DM is diabetic polyneuropathy. The functional status of the trigeminal reflex pathways was reflected through the blink reflex. There were a few existing reports of the relationship between diabetes mellitus and TN- related orofacial pain, which is discussed in this paper.
Introduction
Trigeminal Neuralgia (TN) is the most common type of neuropathic pain in the stomatognathic system. It is difficult to determine the prevalence because the disease is often not recognized as neuralgia so the symptomatology is in dental practice initially related to odontogenic pain of unclear etiology. The complete and definitive diagnostics as well as treatment procedures are the responsibility of a neurologist [1-3].
Also, co-morbidity of TN or pain related to TN and many systemic diseases may induce orofacial pains, whether as an accompanying condition to the main disease (metabolic and endocrine diseases, rheumatic diseases, etc.) or identified as a risk factor (for example, hypertension for TN) or trigeminal pain caused by trauma [4].
Type 2 Diabetes Mellitus (DM) is a heterogeneous group of diseases characterized by different degrees of insulin resistance, disorders of insulin function, and/or secretion with an increase of glucose production in the liver by the process of gluconeogenesis [5].
The most common neurogenic complication of type 2 DM is diabetic polyneuropathy, which is a microvascular complication characterized by progressive sensory loss with or without neuropathic pain. Its typical clinical expression is burning pain, electrical shocks associated with paraesthesia or dysesthesia, and additional allodynia. Risks and coexisting factors for diabetic polyneuropathy are older age, long diabetes duration, poor glycaemic control, higher BMI, nicotinism, alcohol consumption, elevated systolic blood pressure, peripheral vascular disease, and hypercholesterolemia [6-12].
DM is a lifelong incurable disease that also causes psychological disturbances in patients regarding their coping with a specific diet, exercise, and regular insulin intake [13,14]. For TN, the importance of the electro-neurophysiological blink reflex procedure lies in the presentation of neurogenic abnormalities that manifest as dysfunction of the trigeminal sensory system. Besides, the nociceptive blink reflex procedure is particularly important as a diagnostic instrument for TN-related pain disease [15,16].
Diabetic polyneuropathy includes painful sensations of cranial nerves (the third, fourth, sixth, and seventh cranial nerves) [17-19], while the involvement of the fifth (trigeminal) nerve has been described in several clinical cases [20-26]. Hyperglycaemia was previously diagnosed in as many as 33% of patients with TN according to Hindi, et al. [27].
There is a confirmed correlation between glycemic levels, HbA1c, and the progression of late complications in DM, and the level of HbA1c ≤ 7% is defined as the target of good glycemia regulation [28]. There is a clinical correlation between poorly regulated DM expressed by HbA1c interpretation and the manifestation of pain in the orofacial region [29,30].
The aim of this paper is to review orofacial neuralgic pain pathology related to type 2 DM, with a clinical report of a female patient who suffered from diabetic polyneuropathy.
A case report
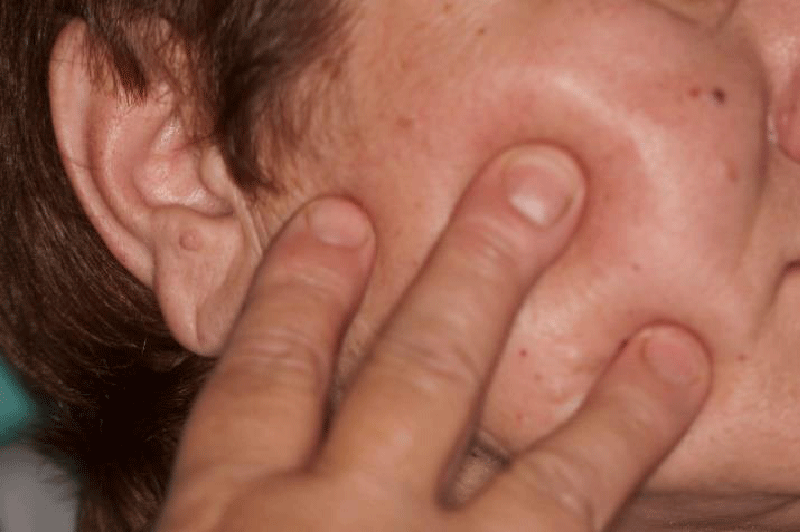
A 61-year-old retired female patient was referred to the Removable Prosthodontic Department, School of Dental Medicine, University of Zagreb, for differential diagnostics of suspect orofacial pain related to Temporomandibular Joint (TMJ) disorder or TN. The recent symptoms were limited mouth opening, and an unpleasant disorder of sensation (burning and heat, electric shock sensation) related to the right side of the face (Figure 1), particularly the second right premolar tooth. One of the indications of TMJ disorder during the neurological examination was the hyperextension of the right condyle seen on the x-ray image of the TMJ.
Patient's medical history
The patient had been regularly taking insulin (DM type 2) since 2000, and previously, since 1995 had been using oral hypoglycemic drugs. Distal diabetic polyneuropathy was diagnosed as early as 2000 at a neurological examination (chronic polyneuropathy diabetic) together with a superimposed radicular lesion involving L5 dermatomes (the fifth lumbar spinal nerve) bilaterally (more dextrally) and S1 (the first sacral spinal nerve) dextrally. Even then she had a sensation of numbness in her lower legs and pain in both heels as well as occasional lumbar-sacral pain with propagation in the right hip. The examination showed that rheumatologic symptoms (and cervical, and chronic lumbosacral) were more pronounced together with diabetes-related symptoms. Lasègue test was negative which excludes herniated discs, in a patient with lower back pain mostly located at L5, S1, or S2. Normal values of bone density were determined by densitometry from 2001 and 2004. Her body mass index was elevated (BMI 29.36 kg/m2). The results of the last diabetological examination prior to a neurological treatment, showed unsuccessful diabetes treatment due to additional, borderline dyslipidemia as well as hypertension (blood pressure was RR 170/80 mm Hg without cardiologic symptoms). The results of laboratory tests are shown in Table 1 [28].
Neurological examination
During a recent neurological examination at a clinic, radiculopathy changes on the level of L5 were confirmed by electromyography. Multi-slice Computerized Tomography (CT) of the brain did not reveal fresh signs of ischemia, hemorrhage, expansive process, or extra-axial collection. Transcranial Doppler sonography and extracranial color Doppler, as well as power Doppler sonography, were within normal ranges regarding the patient’s age. As an abnormal blink reflex finding, latency values were shown in Table 2 [17,18].
Patient’s history related to orofacial pain
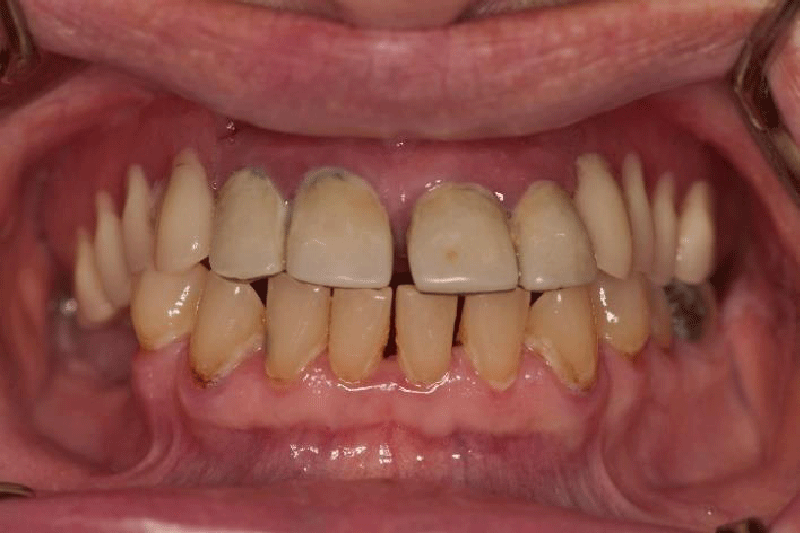
The first orofacial symptoms appeared briefly three years ago when the patient experienced stress due to her husband’s hospitalization. Since then, she had experienced problems with the second right premolar tooth, which she wanted to extract due to an odd and unpleasant electric shock sensation, although there was no obvious odontogenic symptomatology (Figures 2,3). Later on, there were no strong or frequent attacks until five months ago when her brother died. At that time, the burning sensation returned but more intensively along with the sensation of heat and electric shock in the second right premolar tooth. The attacks were more frequent than before and after root canal treatment of the second right premolar and the taking of antibiotics (amoxicillin with clavulanic acid). The radiologic examination excluded odontogenic etiology. Also, the electric shock sensation spread diagonally from the second right premolar tooth towards the ear and the patient felt a burning sensation in the mouth and numbness of the right side of the tongue. In that period, the pain appeared in the morning when she got up and it ceased around 2 pm, and then returned in the evening during rest, for example, when she was watching television. The problems caused limited mouth opening so that she could not eat apples and other food harder to chew, and often she could not drink coffee from a cup or speak. The patient recollected losing at least one or two teeth distally from tooth 44 with similar symptomatology. Prior to neurologic treatment, she visited an oral medicine specialist.
Dental and functional examination
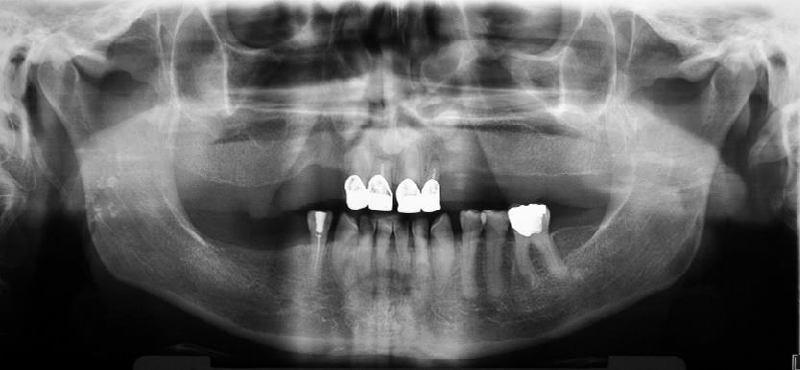
The patient wore an upper partial acrylic denture and crowns on the anterior teeth, which were two years old. The supporting zone of the teeth in the lower right segment was not prosthodontically replaced. The patient had suspected gingivitis and visibly poor oral hygiene, but with a preserved alveolar bone margin which was visible on the panoramic X-ray image. Condyles were symmetrical, and the right styloid process elongated, but the patient denied feeling any pain on swallowing.
Targeted diagnostics excluded symptomatology related to temporomandibular disorders (TMD)
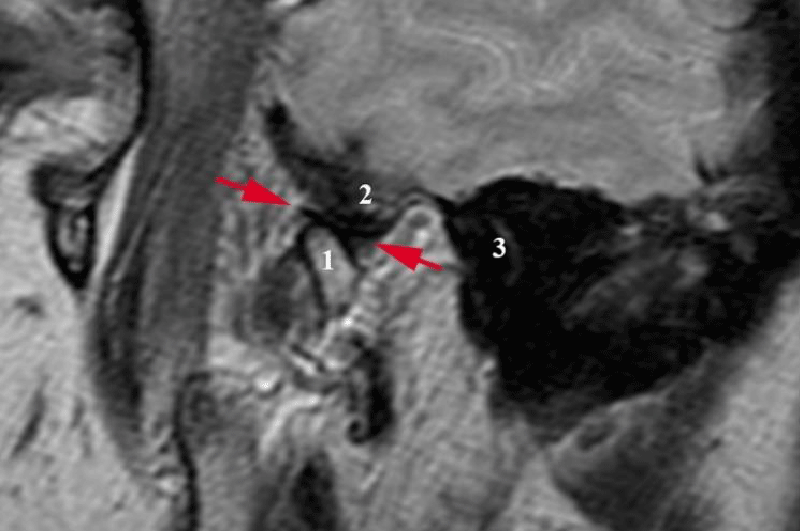
Diagnostic Criteria (DC) for TMD [31] were used as well as manual examination techniques (manual functional analysis) according to Bumann and Groot Landeweer [32]. The clinical examination did not show any pathology related to masticatory muscles and TMJs, there was neither pathologic noise in TMJs, nor any pain on mouth opening (active mouth opening amounts to 48 mm now, the patient states that in the period of symptoms’ manifestation, it amounted to 33 mm or less). On palpation, there is neither pronounced anterior condyle movement, nor any subluxation settling across the tuberculum dextrally. Passive compressions did not provoke pain in the TMJ. Magnetic resonance imaging ("Harmony", Siemens, Erlangen, Germany) was performed by weighted (TR 450/TE 12; matrix 256 x 192; 160 x 160 field of view) and T2 weighted images (TR 3000/TE 66; matrix 389 x 512; 190 x 190 field of view) showed physiologic relations in both TMJs with the hyperextension of both condyles about 1 cm across the zenith of the tuberculum. Both in open and closed mouth positions, the discs were in physiologic relation with the condyles and without any signs of osteoarthritis or effusion of articular spaces (Figure 4).
Psychological measurement
From the patient's history and medical records, it was clear that due to complex painful symptoms, the patient took drugs related to her mental condition. She mentioned that several times, during the TN attacks, she experienced additional stressful events, although she also took several drugs due to her diabetic polyneuropathy: benzodiazepine anxiolytic alprazolam (Helex, 0.5 mg, 1 pill/day); tricyclic antidepressant amitriptyline (Amyzol 25 mg, 2 pills 3 hours before bedtime), as well as an opioid analgesic tramadol (Tramal, 3 x 10 drops, 10 drops = 25 mg).
The psychological assessment was carried out by Spielberger’s State-Trait Anxiety Inventory (STAI) Form Y [33]. STAI is a commonly used measure of self-reporting anxiety on a four-point Likert scale. The range of scores is 20-80, with the higher score indicating greater anxiety. STAI 1 test measures anxiety as a subjective state, a feeling lasting for a week, including the day of testing, and STAI test 2 measures anxiety as a relatively stable individual characteristic during life in general. According to Spielberger for the group of subjects aged 50≥, borderline values for the female gender are: 32.20 for STAI 1 and 31.79 for STAI 2. The patient showed high levels of anxiety on both scales (STAI 1: 46, STAI 2: 58).
Three-years follow-up
After the neurologic and dental treatment, the patient continued the therapy for her basic illness (DM type 2), hyperlipidemia, while the neuralgia was treated with vitamin B only. Mouth opening was painless, amounting to 46 mm, but she still complained about the burning sensation, for example, when eating an apple, which appears in the mandible dextrally and under tooth 44. She also had numbing pain along the zygomatic bone on the right side of the face and towards the right eye. In conclusion, although the severe attack did not recur, the patient’s subjective opinion is that there was no improvement in her condition. The result of the laboratory test for HbA1c was 59 mmol/mol (<42 reference value), which reveals insufficient control of the hyperglycemia, that is, of DM.
Discussion with a literature review
In general, DM is a public health problem, since its prevalence in populations older than 20 is expected to rise from 451 (in 2017) million to 693 million by 2045. DM treatment (that is, the treatment of hyperglycemia) is challenging because it often includes accompanying diseases: obesity (80%), hypertension (60%), and dyslipidemia (30% - 40%) [5].
It is a diagnostic challenge to differentiate nociceptive (somatic) and neuropathic pain and, subsequently, treat it correctly [3]. TMDs are characterized by physiologic activation of nociceptors as a response to musculoskeletal tissue damage (for example, TMJs), whereas neuropathic pain is a lesion of the somatosensory nervous system, thus causing abnormal activity of the nociceptive pathways [1,2]. In the pathophysiology of the genesis of the painful symptoms, both peripheral and central mechanisms have been proposed to play an important role. Damage to peripheral nerves results in hyperexcitability in primary afferent nociceptors (peripheral sensitization) which in turn leads to hyperexcitability in central neurons (central sensitization) and generation of spontaneous impulses within the axon as well as within the dorsal root ganglion of these peripheral nerves [9,10].
According to Alajbegovic, et al. [11] DM duration is the key factor for the development of microvascular complications of neuropathy. In our study, the risk was greater in female patients with type 2 diabetes. The prevalence of diabetic polyneuropathy is considered to be up to 50.8% of patients with DM type 2, depending on the design and sample of the study. 32.8% of patients with diabetic polyneuropathy had neuropathic pain, which was a prevalence of 17.9% for patients with DM type 2. Only 50% of patients with diabetes used analgesic pharmacotherapy, out of which 28% is under recommended treatment for neuropathic pain (anticonvulsants or antidepressants). The opiate derivative tramadol is helpful in the management of painful diabetic peripheral neuropathy, regardless of its risks of addiction [15].
In most cases, cranial nerve involvement in diabetics (range 3% - 14%) relates to motor neuropathies, causing acute onset of ophthalmoplegia. Multiple cranial nerve neuropathies can occur simultaneously in the diabetic population but they can also be the first clinical signs of recently diagnosed diabetes. A clinical manifestation of cranial nerve neuropathies is palsy of involved cranial nerves with an incidence of 0.19% in diabetic patients compared with an incidence of 0.13% in the non-diabetic population [21].
There is a possible relation to poly cranial neuritis characterized by damage to sensory and motor nerves which has been found in diabetics with Ramsay Hunt syndrome, which manifests as a sensory and motor complication of herpes zoster infection, including the trigeminal nerve [34]. Tu, et al. [21] presented a clinical case of multiple cranial neuropathies with four episodes of oculomotor and facial nerve palsy, involving the left oculomotor, trochlear nerve, and possible ophthalmic division of trigeminal nerve.
Trigeminal nerve appears to be rarely involved in patients with diabetes mellitus. Cruccu, et al. [23], Urban, et al. [24], Wong, et al. [25], and Takayama, et al. [26] are examples of very few existing reports of the relationship between diabetes and TN- related orofacial pain. Cruccu, et al. [23] found sensory trigeminal neuropathy in 8 out of 15 diabetic patients with chronic inflammatory demyelinating polyneuropathy, which causes subclinical neuropathy to its mandibular branch. Urban, et al. [24] researched trigeminal and facial nerve functions electrophysiologically and concluded that their involvement in diabetes-related polyneuropathy can be expected, although limb nerve involvement is significantly more frequent.
Wong, et al. [25] described the involvement of the oculomotor nerve and the maxillary division of the trigeminal nerve in a case of a 53-year-old man who suffered from pain and swelling in the right infraorbital region in the area of the right maxillary canine. He had poorly controlled insulin-dependent diabetes mellitus complicated by peripheral neuropathy, hypertension, and migraine headaches. Contrary to the satisfactory CT findings of the patient described in our paper, in the above-mentioned case, the CT showed generalized atrophy and a focal region of low attenuation in the left frontal lobe and central pons. The left frontal lobe attenuation was consistent with encephalomalacia and likely represented a previous infarction. Takayama, et al. [26] described bilateral trigeminal nerve involvement in a 69-year-old male patient with DM type 2. Neuropathy of ophthalmic and maxillary branches was characterized by causalgia and dysaesthesia in the patient's cheeks and around his eyes. Attack episodes were related to the level of successful glycemic control.
Patients with chronic inflammatory demyelinating polyneuropathy were a sample for research in the study of Kokubun and Hirata [35], and using neurophysiological tests, they found that subclinical trigeminal and facial neuropathies were extremely high (60% - 85%).
The best indicator of metabolic control is glycosylated hemoglobin (HbA1c), which is a fraction of hemoglobin to which the glucose from the blood bonds independently of insulin; hence, it is directly dependent on the average level of glucose in blood [28]. HbA1c value shows the success of diabetes control in the previous two to three months. The laboratory test results (HbA1c) of the reported case of the female patient did not show successful control of DM type 2. However, Takayama, et al. [26] noted that symptoms had become more severe when HbA1c increased to as high as 89 mmol/mol. When the patient took insulin therapy, the level of HbA1c decreased to 51 mmol/mol. They concluded that better glycaemic control improved the patient’s clinical picture related to neuropathic pain.
The finding of the blink reflex test of this patient supports the diagnosis of trigeminal neuropathy, which shows irritative changes to the afferent branch of the reflex arc dextrally [36]. Mikula, et al. [37] used the blink reflex method and concluded that it can be useful in differential diagnostics: the BR may prove a significant aid in distinguishing the idiopathic TN (normal latencies R2 and R2c) and symptomatic (prolonged latencies R2 and R2c) disease types. The incidence of the R3 component was higher (84%) in patients with idiopathic TN than in patients who suffered from symptomatic TN (20%).
The biopsychosocial conceptualization of the pain experience recognized psychological factors as a part of the multidimensional description of pain; especially chronic pain conditions such as diabetic polyneuropathy. Since DM type 2 is a chronic disease, apart from timely diagnosing, regular controls with treatment modifications if necessary, a psychosocial factor of facing the patient with the disease and accepting a healthy lifestyle is very important [15]. Anxiety is the most common affective disorder and a great problem for patients with DM type 2 because as many as 19.8% of them have a diagnosis of anxiety disorders [38]. In DM treatment, psychological evaluation is necessary because psychological management improves quality of life, and has positive impacts, particularly on painful diabetic polyneuropathy. Successful treatment of idiopathic TN is challenging. There is no gold standard for assessing neuropathic pain caused by idiopathic TN. In order to successfully treat this type of orofacial pain, which can be characterized as severe pain without a clear etiopathogenesis, a collaboration between the dentist and the neurologist and active participation from the patient’s side are needed [39]. Although the dentist is not the primary health care provider who decides on the choice of pharmaceutical therapy, the intensity of symptoms in the stomatognathic system should be taken into account, in particular the involvement of toothache and intensity of neuralgic attacks without a dental cause.
Conclusion
Sensory complaints in the area of the teeth, jaws, and mouth often escape notice or remain undiagnosed. A multidisciplinary approach is needed for a proper understanding of the causes as well as for treatment planning in cases of possible co-morbidity with orofacial neuropathic and/or nociceptive pain. Beyond the scope of dental medicine, and biochemical diagnostics, HbA1c has helped in the interpretation of both therapeutic efficacy and the risk of persistent diabetic complications, such as in cases of neuralgiform pain of the orofacial region. In addition, the blink reflex method can be a useful diagnostic tool to distinguish asymptomatic patients from symptomatic patients with TN.
- Costa YM, Karlsson P, Bonjardim LR, Conti PCR, Tankisi H, Jensen TS, Nyengaard JR, Svensson P, Baad-Hansen L. Trigeminal nociceptive function and oral somatosensory functional and structural assessment in patients with diabetic peripheral neuropathy. Sci Rep. 2019 Jan 17;9(1):169. doi: 10.1038/s41598-018-37041-4. PMID: 30655584; PMCID: PMC6336810.
- Xu Z, Zhang P, Long L, He H, Zhang J, Sun S. Diabetes mellitus in classical trigeminal neuralgia: A predisposing factor for its development. Clin Neurol Neurosurg. 2016 Dec;151:70-72. doi: 10.1016/j.clineuro.2016.10.015. Epub 2016 Oct 22. PMID: 27816028.
- Badel T, Bašić Kes V, Jerolimov V, Zadravec D, Savić-Pavičin I, Anić Milošević S. EVAULATION OF BLINK REFLEX BETWEEN PATIENTS WITH IDIOPATHIC TRIGEMINAL NEURALGIA AND HEALTHY VOLUNTEERS. Acta Clin Croat. 2022 Sep;61(Suppl 2):121-128. doi: 10.20471/acc.2022.61.s2.16. PMID: 36824643; PMCID: PMC9942457.
- Benoliel R, Kahn J, Eliav E. Peripheral painful traumatic trigeminal neuropathies. Oral Dis. 2012 May;18(4):317-32. doi: 10.1111/j.1601-0825.2011.01883.x. Epub 2011 Dec 27. PMID: 22212350.
- Amadou-Diaw N, Braud A, Boucher Y. Persistent, neuropathic-like trigeminal pain after dental implant loading. J Clin Exp Dent. 2022 Feb 1;14(2):e185-e191. doi: 10.4317/jced.59248. PMID: 35173902; PMCID: PMC8842286.
- Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018 Apr;138:271-281. doi: 10.1016/j.diabres.2018.02.023. Epub 2018 Feb 26. PMID: 29496507.
- Hafez D, Nelson DB, Martin EG, Cohen AJ, Northway R, Kullgren JT. Understanding type 2 diabetes mellitus screening practices among primary care physicians: a qualitative chart-stimulated recall study. BMC Fam Pract. 2017 Apr 4;18(1):50. doi: 10.1186/s12875-017-0623-3. PMID: 28376802; PMCID: PMC5381083.
- Knopp M, Rajabally YA. Common and less common peripheral nerve disorders associated with diabetes. Curr Diabetes Rev. 2012 May;8(3):229-36. doi: 10.2174/157339912800564034. PMID: 22283678.
- Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: epidemiology, natural history, early diagnosis, and treatment options. Pain Med. 2008 Sep;9(6):660-74. doi: 10.1111/j.1526-4637.2007.00347.x. PMID: 18828198.
- Wang P, Liu B, Rong T, Wu B. Is diabetes the risk factor for poor neurological recovery after cervical spine surgery? A review of the literature. Eur J Med Res. 2022 Nov 23;27(1):263. doi: 10.1186/s40001-022-00879-6. PMID: 36419189; PMCID: PMC9686083.
- Alajbegovic A, Alajbegovic S, Resic H. Diabetic neuropathy assessed at two-time points five years apart. Diabetologia Croatica 2008; 37: 91-96.
- Gupta G, Massie R, Doherty TJ, Bourque PR, Radhakrishna M, Finlayson RJ, Besemann M, Simantirakis E, Dyck PJB. Diabetic Cranio-Cervico-Radiculoplexus Neuropathy. PM R. 2015 Nov;7(11):1189-1193. doi: 10.1016/j.pmrj.2015.05.003. Epub 2015 May 12. PMID: 25978945.
- Jurisić-Erzen D, Benko K, Ljubić S, Jerković R. The prevalence of depression and anxiety in seafarers type 2 diabetic patients. Coll Antropol. 2011 Dec;35(4):1067-70. PMID: 22397240.
- Borovcanin MM, Vesic K, Petrovic I, Jovanovic IP, Mijailović NR. Diabetes mellitus type 2 as an underlying, comorbid or consequent state of mental disorders. World J Diabetes. 2023 May 15;14(5):481-493. doi: 10.4239/wjd.v14.i5.481. PMID: 37273248; PMCID: PMC10236997.
- Van Acker K, Bouhassira D, De Bacquer D, Weiss S, Matthys K, Raemen H, Mathieu C, Colin IM. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab. 2009 Jun;35(3):206-13. doi: 10.1016/j.diabet.2008.11.004. Epub 2009 Mar 17. PMID: 19297223.
- Bago I, Bošnjak A. Relationship of HbA1c level and smoking to periodontal status in insulin-dependent diabetic patients. Acta Stomatol Croat. 2006; 40: 248-255.
- Jerath N, Kimura J. F wave, A wave, H reflex, and blink reflex. Handb Clin Neurol. 2019;160:225-239. doi: 10.1016/B978-0-444-64032-1.00015-1. PMID: 31277850.
- Valls-Sole J. Spontaneous, Voluntary, and Reflex Blinking in Clinical Practice. J Clin Neurophysiol. 2019 Nov;36(6):415-421. doi: 10.1097/WNP.0000000000000561. PMID: 31688324.
- Gupta M, Chitneni A, Ghorayeb J, Schnetzer B, Klusek M. Cervical Spinal Cord Stimulation for Trigeminal Neuralgia: a Narrative Review. Curr Pain Headache Rep. 2022 Aug;26(8):639-645. doi: 10.1007/s11916-022-01066-2. Epub 2022 Jun 18. PMID: 35716273.
- Papanas N, Heliopoulos I, Piperidou H, Maltezos E. Simultaneous, painless, homolateral oculomotor and trochlear nerve palsies in a patient with type 2 diabetes mellitus. Neuropathy or brainstem infarction? Acta Diabetol. 2006 May;43(1):19-21. doi: 10.1007/s00592-006-0205-7. PMID: 16710645.
- Tu MC, Chang YY, Lin TK. Recurrent multiple cranial neuropathies in a diabetic patient. Acta Neurol Taiwan. 2010 Sep;19(3):208-12. PMID: 20824543.
- Isagulyan E, Tkachenko V, Semenov D, Asriyants S, Dorokhov E, Makashova E, Aslakhanova K, Tomskiy A. The Effectiveness of Various Types of Electrical Stimulation of the Spinal Cord for Chronic Pain in Patients with Postherpetic Neuralgia: A Literature Review. Pain Res Manag. 2023 Mar 24;2023:6015680. doi: 10.1155/2023/6015680. PMID: 37007861; PMCID: PMC10065853.
- Cruccu G, Agostino R, Inghilleri M, Innocenti P, Romaniello A, Manfredi M. Mandibular nerve involvement in diabetic polyneuropathy and chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 1998 Dec;21(12):1673-9. doi: 10.1002/(sici)1097-4598(199812)21:12<1673::aid-mus8>3.0.co;2-a. PMID: 9843068.
- Urban PP, Forst T, Lenfers M, Koehler J, Connemann BJ, Beyer J. Incidence of subclinical trigeminal and facial nerve involvement in diabetes mellitus. Electromyogr Clin Neurophysiol. 1999 Jul-Aug;39(5):267-72. PMID: 10421997.
- Wong AD, Best JA, Shapiro RD. Diabetic polyneuropathy involving the maxillofacial region: a case report. J Oral Maxillofac Surg. 2002 Aug;60(8):935-8. doi: 10.1053/joms.2002.33866. PMID: 12149742.
- Takayama S, Osawa M, Takahashi Y, Iwamoto Y. Painful neuropathy with trigeminal nerve involvement in type 2 diabetes. J Int Med Res. 2006 Jan-Feb;34(1):115-8. doi: 10.1177/147323000603400115. PMID: 16604832.
- Hindi E, Almusally BA, Bawareth R, Alhamadah W, Alfaraj R, Almwled A, Mousa A, Saga M. Diabetic Polyneuropathy in Type 1 and Type 2 Diabetes Mellitus: A Cross-Sectional Study. Cureus. 2022 Oct 6;14(10):e30004. doi: 10.7759/cureus.30004. PMID: 36348847; PMCID: PMC9637022.
- Arnardóttir E, Sigurðardóttir ÁK, Graue M, Kolltveit BH, Skinner T. Using HbA1c measurements and the Finnish Diabetes Risk Score to identify undiagnosed individuals and those at risk of diabetes in primary care. BMC Public Health. 2023 Jan 31;23(1):211. doi: 10.1186/s12889-023-15122-y. PMID: 36721135; PMCID: PMC9887861.
- Bokan-Mirković V, Škarić-Karanikić Ž, Nejkov S, Vuković M, Ćirović D. Diabetic Polyneuropathy and Risk of Falls: Fear of Falling and Other Factors. Acta Clin Croat. 2017 Dec;56(4):721-727. doi: 10.20471/acc.2017.56.04.20. PMID: 29590728.
- Zhang A, Zhang W, Xu H, Guo C, Yuan L, Xu Y, Ren J, Min L, Sun Q, Lou M, Wei L, Lin S. Diabetes mellitus contributes to carbamazepine resistance in patient with trigeminal neuralgia. Neurosurg Rev. 2021 Apr;44(2):1119-1125. doi: 10.1007/s10143-020-01304-4. Epub 2020 Apr 24. PMID: 32333283.
- Türp JC. Vorstellung der Achse-I-Klassifikation. Craniomand Func. 2014; 6:231-9.
- Bumann A, Lotzmann. TMJ Disorders and Orofacial Pain - The Role of Dentistry in a Multidisciplinary Diagnostic Approach. Thieme, Stuttgart - New York. 2002.
- Spielberger CD. Manual for the State-Trait Anxiety Inventory [in Croatian] Jastrebarsko: Slap; 2001.
- Sun WL, Yan JL, Chen LL. Ramsay Hunt syndrome with unilateral polyneuropathy involving cranial nerves V, VII, VIII, and XII in a diabetic patient. Quintessence Int. 2011 Nov-Dec;42(10):873-7. PMID: 22026001.
- Kokubun N, Hirata K. Neurophysiological evaluation of trigeminal and facial nerves in patients with chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 2007 Feb;35(2):203-7. doi: 10.1002/mus.20679. PMID: 17063459.
- Badel T, Bašić Kes V, Jerolimov V, Zadravec D, Savić-Pavičin I, Anić Milošević S. EVAULATION OF BLINK REFLEX BETWEEN PATIENTS WITH IDIOPATHIC TRIGEMINAL NEURALGIA AND HEALTHY VOLUNTEERS. Acta Clin Croat. 2022 Sep;61(Suppl 2):121-128. doi: 10.20471/acc.2022.61.s2.16. PMID: 36824643; PMCID: PMC9942457.
- Mikula I, Trkanjec Z, Negovetić R, Miskov S, Demarin V. Differences of blink-reflex abnormalities in patients suffering from idiopathic and symptomatic trigeminal neuralgia. Wien Klin Wochenschr. 2005 Jun;117(11-12):417-22. doi: 10.1007/s00508-005-0364-5. PMID: 16053198.
- Han B, Wang L, Zhang Y, Gu L, Yuan W, Cao W. Baseline anxiety disorders are associated with progression of diabetic kidney disease in type 2 diabetes. Ren Fail. 2023 Dec;45(1):2159431. doi: 10.1080/0886022X.2022.2159431. PMID: 36632821; PMCID: PMC9848365.
- Di Stefano G, La Cesa S, Truini A, Cruccu G. Natural history and outcome of 200 outpatients with classical trigeminal neuralgia treated with carbamazepine or oxcarbazepine in a tertiary centre for neuropathic pain. J Headache Pain. 2014 Jun 9;15(1):34. doi: 10.1186/1129-2377-15-34. PMID: 24912658; PMCID: PMC4067104.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
