Journal of Dental Problems and Solutions
Study about the Effect of Total Flavonoids in Toothpaste on Bacteriostasis and Hemostasis
School of Medicine & Health Sciences, Guangzhou Huashang College, Guangzhou, Guangdong 511328, China
Author and article information
Cite this as
Zou J, Tan J, Li Q, Zhang W, Wang R. Study about the Effect of Total Flavonoids in Toothpaste on Bacteriostasis and Hemostasis. J Dent Probl Solut. 2025;12(2):009-013. Available from: 10.17352/2394-8418.000132
Copyright License
© 2025 Zou J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Abstract
This study is based on the extraction process of total flavonoids in traditional Chinese medicine compound, with ethanol concentration, extraction time and solid-liquid ratio as variables, three factors and three levels orthogonal experiments were conducted. We used the heat reflux extraction method to concentrate the extract. The total flavonoid content was determined using ultraviolet spectrophotometry. The optimum extraction conditions were determined using the total flavonoid content as the index. According to the experimental results, the concentration of total flavonoids in the range of 0. 0096~0. 0576 mg/ml showed a good linear relationship with absorbance. Therefore, the optimal process conditions were identified as A1B3C3: a 95% ethanol concentration; an extraction time of 120 minutes; a material-to-liquid ratio of 1:12; and a total flavonoid content of 8.273%. Orthogonal test can be used to analyze the extraction process of antibacterial components.
Main article text
1. Introduction
At present, some toothpastes claim to have exclusive secret recipes with bacteriostatic and hemostatic properties. These toothpastes are reported to contain a substance called tranexamic acid [1-3]. This kind of substance belongs to a class of synthetic pharmaceutical ingredients which are effective in bacteriostasis and hemostasis [4-10], but can cause adverse reactions in pregnant women [11,12]. Therefore, the development of toothpaste made from pure natural plant ingredients (pure Chinese herbal ingredients) is particularly important.
It has been shown that flavonoids have bacteriostatic effect [13-19], are found in a variety of natural plants and can be used as the main ingredients in compound Chinese medicine [20-34]. It has different inhibitory effects on many microorganisms, including gram-positive bacteria, gram-negative bacteria and fungi [35,36].
In this study, an optimum extraction protocol for flavonoids from a compound Chinese medicine was established. The resulting flavonoid-rich extract was then blended with validated active constituents, and the antibacterial efficacy of the formulation in toothpaste was subsequently investigated.
2. Materials and methods
2.1 Instrument
Ultraviolet visible spectrophotometer (UV6000 SHANGHAI METASH INSTRUMENTS CO., LTD); Electronic balance (FA2004 CHANGZHOU LUCKY ELECTRONIC EQUIPMENT Co., Ltd); Circulating water vacuum pump (SHZ-DS (Ⅲ) Zhengzhou Boke Instrument Equipment Co., Ltd); Rotary evaporator (SY-2000 SHANGHAI YA RONG BIOCHEMISTRY INSTRUMENT FACTORY); Centrifuge (TDL-50B SHANGHAI ANTING Electrical & Marine Instruments Co., Ltd); CNC ultrasonic cleaner (KQ5200DE Kun Shan Ultrasonic Instruments Co., Ltd); Sleeve thermostat (TC-15 HAINING XINHUA MEDICAL INSTRUMENT CO.,LTD)
2.2 Reagent
Astragalus; Bupleurum; Magnolia officinalis; Raw pinellia; Paris polyphylla; Centipede; Zedoary turmeric; 95% Ethanol (Fuyu Fine Chemical Co., Ltd); Carbinol (Fuyu Fine Chemical Co., Ltd); Sodium nitrite (Tianjin Damao Chemical Reagent Factory); Aluminium nitrate (Guangzhou Chemical Reagent Factory); Sodium hydroxide (Fuchen Chemical Reagent Factory); Rutin standard (National Institutes for Food and Drug Control); Purified water.
2.3 Compatibility and treatment of compound medicinal materials
1.3.1 Compatibility of compound medicinal materials: Astragalus 15.00 g; Bupleurum 7.50 g; Magnolia officinalis 5.00 g; Raw pinellia 10.00 g; Paris polyphylla 7.50 g; Centipede 5.00 g; Zedoary turmeric 7.50 g.
Weigh out all the medicinal materials according to the formula: Astragalus 15.00 g; Bupleurum 7.50 g; Magnolia officinalis 5.00 g; Raw pinellia 10.00 g; Paris polyphylla 7.50 g; Centipede 5.00 g; Zedoary turmeric 7.50 g. Mix and crush them in the pulveriser for 5 seconds, then remove the crushed materials to use as the experimental preparation.
2.4 Orthogonal test
In order to explore the extraction efficiency of the total flavonoids of the traditional Chinese medicine compound under different conditions, the heat reflux extraction method is adopted, at the same time, three variables including ethanol concentration (A), extraction time (B) and solid-liquid ratio (C) were selected, and three levels of each factor were selected to design orthogonal experiment. The experiment was arranged according to the orthogonal table, and the process was optimized with the content of total flavonoids as the index [37-42].
Table 1 shows the factor variables and Table 2 shows the orthogonal experiments.
2.5 Standard curve preparation
2.5.1 Preparation of rutin reference solution: Exactly 12 mg of dried rutin reference standard was weighed into a 50 mL volumetric flask. An appropriate volume of methanol was then added, and the mixture was gently heated on a water bath until complete dissolution was achieved. Once cooled to room temperature, the volume was adjusted to 50 mL with water, and the solution was thoroughly mixed to yield a rutin concentration of 0.24 mg mL-1 [43].
2.5.2 Selection of absorption wavelength and preparation of standard curve:
- Aliquots of 1.0, 2.0, 3.0, 4.0, 5.0 and 6.0 mL of rutin reference solution were transferred into separate 25 mL volumetric flasks, 6.0 mL of distilled water and 1.0 mL of 5% (w/v) sodium nitrite solution were added, and each mixture was homogenised and allowed to stand for 6 min.
- Subsequently, 1.0 mL of 10% (w/v) aluminium nitrate solution was added to each flask, the solutions were shaken and left to stand for another 6 min.
- Thereafter, 10.0 mL of 4% (w/v) sodium hydroxide solution was introduced into each flask, the volumes were brought to 25 mL with distilled water, and the solutions were thoroughly mixed and left to stand for 15 min.
- A blank was prepared using all reagents except rutin, and the maximum absorption peak at 510 nm was determined by spectral scanning over the range 200–800 nm.
- Using the blank as the reference, the absorbance of each of the six standard solutions was measured at 510 nm, and a calibration curve relating concentration to absorbance was constructed from the obtained data.
2.6 Determination of total flavonoid content
Exactly 0.2000 g of the compound extract was weighed into a 10 mL centrifuge tube, 10 mL of methanol was added, and the mixture was sonicated at 30 °C until complete dissolution was achieved. The solution was centrifuged at 1000 rpm for 3 min, and the supernatant was collected as the test solution. Aliquots (1.5 mL) of the test solution were transferred into 25 mL volumetric flasks, 6.0 mL of water and 1.0 mL of 5% (w/v) sodium nitrite solution were added, and the mixture was homogenised and allowed to stand for 6 min. Subsequently, 1.0 mL of 10% (w/v) aluminium nitrate solution was introduced, the flask was shaken, and the solution was left for another 6 min. Finally, 10.0 mL of 4% (w/v) sodium hydroxide solution was added, the volume was adjusted to 25 mL with water, the contents were mixed thoroughly, and the solution was left to stand for 15 min. The absorbance was measured at 510 nm, and the total flavonoid content was calculated.
2.7 Methodological investigation
2.7.1 Precision test: For each group, 1.5 mL of the test solution was accurately pipetted in six replicates, reagents were sequentially added according to the standard-curve preparation method to yield 25 mL samples, the absorbance was measured at 510 nm, and the RSD of each group was calculated.
2.7.2 Stability test: A 1.5 mL aliquot of each group’s solution was taken and a 25 mL sample solution was prepared according to the aforementioned method. Absorbances were measured at 510 nm after reaction times of 0, 15, 30, 45 and 60 min, and the RSD was subsequently calculated.
3. Results
3.1 Standard curve
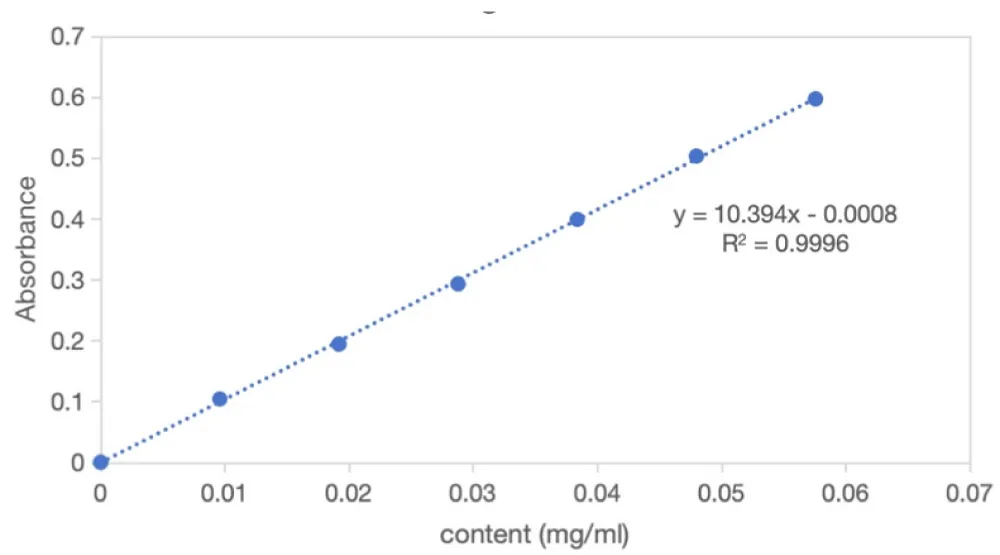
Following the procedure described in Section 2.5.2, six sets of concentrations and corresponding absorbance were obtained. A standard curve was constructed by plotting concentration on the abscissa and absorbance (A) on the ordinate. The linear regression equation of the straight line was y = 10.394x − 0.0008, with a correlation coefficient r = 0.9998 (Figure 1 and Table 3).
The results indicate that total flavonoids exhibited a good linear relationship with absorbance over the concentration range of 0.0096–0.0576 mg mL-1 (Figure 1 and Table 3).
3.2 Determination of total flavonoids
In accordance with the procedure described in Section 2.6, the absorbance of nine groups of test samples were determined in triplicate. These absorbance values were then substituted into the calibration curve established in Section 3.1 to calculate the flavonoid contents. The mean total flavonoid content of each group, obtained from the three parallel determinations, is recorded in Table 4.
3.3 Methodological investigation
3.3.1 Precision test: Following the protocol outlined in Section 2.7.1, six replicate measurements were collected for each of the nine groups, and the precision was evaluated. As shown in Table 5, the RSD values were all below 2%, indicating good precision of the method.
3.3.2 Stability test: In accordance with Section 2.7.2, absorbances at 510 nm were recorded at 0, 15, 30, 45 and 60 min for each of the nine groups, and the corresponding RSD values were calculated. The results in Table 6 demonstrated that the assay possessed good stability.
4. Discussion
The extraction method was precise but only moderately stable. This was attributed to the relatively low stability of total flavonoids under strongly alkaline conditions. Flavonoids are also reduced in stability by light, so the extract should be stored away from light. The reducing agent sodium nitrite also affects the stability of flavonoids to some extent.
This study provides critical technical support for the development and application of natural antimicrobial constituents by systematically optimising the extraction process for total flavonoids from the traditional Chinese medicine compound formula. Under the optimised conditions of 95% ethanol, a 120-minute extraction time and a solid-liquid ratio of 1:12, the experimental data demonstrated a total flavonoid extraction yield of 8.This represents a significant improvement on conventional procedures and confirms the efficacy of orthogonal experimental design for process optimisation in complex systems. It also elucidates the scientific principle that ethanol concentration is the pivotal factor and provides a reproducible framework of parameters for subsequent industrial-scale production.
While the study revealed limited stability of flavonoids under strongly alkaline conditions, this finding clearly indicates the need for further formulation optimisation, suggesting that pH adjustment or antioxidant protection should be incorporated into subsequent product development.
From an application perspective, this study provides a robust scientific basis for developing an 'all-herbal toothpaste'. By systematically screening the compatibility of seven medicinal herbs, including Astragalus and Bupleurum, the study substantiates the antibacterial potential of the composite formula's flavonoid-rich fractions. This innovative, evidence-based solution addresses ongoing concerns about synthetic additives in commercial oral care products. The extraction and analytical methodology developed in this study can also be used as a reference for modernising the development of bioactive constituents from other Traditional Chinese Medicines (TCM), accelerating TCM modernisation research.
5. Conclusion
The effects of ethanol concentration, extraction time, and the solid-to-liquid ratio on the extraction rate of total flavonoids were studied using an orthogonal experiment. Spectral analysis revealed that both the test solution and the rutin control solution absorbed at 510 nm, with the rutin control solution demonstrating a strong linear relationship between absorbance and concentration. Therefore, rutin was selected as the control product to determine the total flavonoid content in the antibacterial compound. The results showed that ethanol concentration was the main factor affecting the extraction rate, followed by extraction time and the solid-to-liquid ratio, and the interaction patterns of these parameters were elucidated. The optimal extraction conditions were a 95% ethanol concentration, an extraction time of 120 minutes and a solid-to-liquid ratio of 1:12. Under these conditions, the extraction rate of total flavones was 8.273%. The extraction method demonstrated good precision and stability.
Acknowledgement
This study was supported by the innovative Research Team Project (Natural Sciences) of Guangdong Provincial General Universities – “Natural Green Cosmetics Innovation Team” (Grant No. 2024KCXTD030).
References
- Lu P, Li M, Lou YC, Su F, Li H, Zhao X, et al. Antiproliferative effects of n-butyl-β-D-fructofuranoside from Kangaisan on Bel-7402 cells. Indian J Pharmacol. 2014;46(1):69–75. Available from: https://doi.org/10.4103/0253-7613.125175
- Wang R, Zhou M, Wang S, Liao Y, Fan X, Ma Y. Determination of tranexamic acid in essential cosmetics by pre-column derivatization capillary electrophoresis coupled with electrogenerated chemiluminescence detection. Chin J Chromatogr. 2020;7:968–74. Available from: https://doi.org/10.3724/sp.j.1123.2019.12002
- Tateyama-Makino R, Abe-Yutori M, Iwamoto T, Tsutsumi K, Tsuji M, Morishita S, et al. The inhibitory effects of toothpaste and mouthwash ingredients on the interaction between the SARS-CoV-2 spike protein and ACE2, and the protease activity of TMPRSS2 in vitro. PLoS One. 2021;16(9):e0257705. Available from: https://doi.org/10.1371/journal.pone.0257705
- Denry I, Nédélec J, Holloway J. Tranexamic acid-loaded hemostatic nanoclay microsphere frameworks. J Biomed Mater Res B Appl Biomater. 2021;1–9. Available from: https://doi.org/10.1002/jbm.b.34918
- Darakhshan S, Goudarzi F, Mirzaie S, Karami A, Kiani A. Hemostatic polyurethane sponge containing kaolin, tannic acid, and tranexamic acid. Polym Eng Sci. 2023;63(9):3013–24. Available from: https://doi.org/10.1002/pen.26424
- Franchini M, Focosi D, Mannuccio Mannucci P. Tranexamic acid: an evergreen hemostatic agent. Semin Thromb Hemost. 2024;50(5):733–8. Available from: https://doi.org/10.1055/s-0044-1779632
- Lam T, Medcalf R, Cloud G, Myles P, Keragala C. Tranexamic acid for haemostasis and beyond: does dose matter? Thromb J. 2023;21:94. Available from: https://doi.org/10.1186/s12959-023-00540-0
- Benjumea A, Díaz-Navarro M, Gago-Campos A, Visedo A, Hafian R, Cercenado E, et al. Validation of the antibacterial effect of topically applied tranexamic acid using in vitro and in vivo models. Front Microbiol. 2024;15:1–9. Available from: https://doi.org/10.3389/fmicb.2024.1367884
- Alissa M, Hjazi A, Abusalim G, Aloraini G, Alghamdi S, Rizg W, et al. Development and optimization of a novel lozenge containing a metronidazole-peppermint oil-tranexamic acid self-nanoemulsified delivery system to be used after dental extraction: in vitro evaluation and in vivo appraisal. Pharmaceutics. 2023;15(9):2342. Available from: https://doi.org/10.3390/pharmaceutics15092342
- Briggs G, Balogh Z. Tranexamic acid and inflammation in trauma. ANZ J Surg. 2020;90(4):426–8. Available from: https://doi.org/10.1111/ans.15755
- Imbesi S, Nettis E, Minciullo P, Leo E, Saija A, Vacca A, et al. Hypersensitivity to tranexamic acid: a wide spectrum of adverse reactions. Int J Clin Pharm. 2010;32:416–9. Available from: https://doi.org/10.1007/s11096-010-9415-8
- Levy YH. Hemostatic agents and their safety. J Cardiothorac Vasc Anesth. 1999;13(4 Suppl 1):6–11. Available from: https://pubmed.ncbi.nlm.nih.gov/10468243/
- Sarbu L, Bahrin L, Babii C, Stefan M, Birsa M. Synthetic flavonoids with antimicrobial activity: a review. J Appl Microbiol. 2019;127(5):1282–90. Available from: https://doi.org/10.1111/jam.14271
- Mao S, Ren Y, Ye X, Tian J. The metabolites of flavonoids with a typical structure enhance bioactivity through gut microbiota. Food Biosci. 2024;59:104165. Available from: http://dx.doi.org/10.1016/j.fbio.2024.104165
- Wu D, Hao L, Liu X, Li X, Zhao G. Comparative transcriptomics reveals the mechanism of antibacterial activity of fruit-derived dihydrochalcone flavonoids against Porphyromonas gingivalis. Food Funct. 2024;19:9734–49. Available from: https://pubs.rsc.org/en/content/articlelanding/2024/fo/d4fo02854f
- Cascaes M, Guilhon G, Zoghbi M, Andrade E, Santos E, Silva J, et al. Antioxidant potential and antimicrobial activity of Myrcia rufipila McVaugh leaves (Myrtaceae). Nat Prod Res. 2021;35(10):1717–22. Available from: https://doi.org/10.1080/14786419.2019.1629912
- Lin Z, Lin Y, Zhang Z, Shen J, Yang C, Jiang M, et al. Systematic analysis of the bacteriostatic mechanism of flavonoids using transcriptome and its therapeutic effect on vaginitis. Aging (Albany NY). 2020;12(7):6292–305. Available from: https://doi.org/10.18632/aging.103024
- Cataneo A, Ávila E, Mendes L, Oliveira V, Ferraz C, Almeida M, et al. Flavonoids as molecules with anti-Zika virus activity. Front Microbiol. 2021;12:710359. Available from: https://doi.org/10.3389/fmicb.2021.710359
- Jo S, Kim S, Kim D, Kim M, Shin D. Flavonoids with inhibitory activity against SARS-CoV-2 3CLpro. J Enzyme Inhib Med Chem. 2020;35:1539–44. Available from: https://doi.org/10.1080/14756366.2020.1801672
- Zhang W, Wang J, Chen Y, Zheng H, Xie B, Sun Z. Flavonoid compounds and antibacterial mechanisms of different parts of white guava (Psidium guajava L. cv. Pearl). Nat Prod Res. 2020;34(11):1621–5. Available from: https://doi.org/10.1080/14786419.2018.1522313
- Al-Jadidi HSK, Hossain MA. Determination of the total phenols, flavonoids, and antimicrobial activity of the crude extracts from locally grown neem stems. Asian Pac J Trop Dis. 2016;5(6):376–9. Available from: https://doi.org/10.1016/S2222-1808(15)61051-9
- Nguyen T, Tran C, Vuong C, Do T, Le T, Mai D, et al. Flavonoids with hepatoprotective activity from the leaves of Cleome viscosa L. Nat Prod Res. 2017;31(22):2587–92. Available from: https://doi.org/10.1080/14786419.2017.1283497
- Yang J, Ma Y, Liu Y, Peng X, Chen X. A comprehensive review of natural flavonoids with anti-SARS-CoV-2 activity. Molecules. 2023;28(6):2735. Available from: https://doi.org/10.3390/molecules28062735
- Pontes M, Torres-Rêgo M, Aquino A, Aquino N, Teles Y, Ximenes R, et al. Benth H.: A prolific source of bioactive flavonoids with antiophidic potential. Phytochem Lett. 2021;41:158–67. Available from: https://doi.org/10.1016/j.phytol.2020.09.025
- Kotkar H, Mendki P, Sadan S, Jha S, Upasani S, Maheshwari V. Antimicrobial and pesticidal activity of partially purified flavonoids of Annona squamosa. Pest Manag Sci. 2001;58(1):33–7. Available from: https://doi.org/10.1002/ps.414
- Al-Tannak N, Al-Hasawi N, Novotny L. UHPLC-UV analysis of morin and structurally related flavonoids with potential anticancer activity. Curr Pharm Anal. 2019;15(4):295–304. Available from: https://doi.org/10.2174/1573412914666171220154224
- Yuan R, Li C, Pan Y, Zhang Z, Zhu Y, Nie Y. Antibacterial and hypoglycemic activity of flavonoids in etiolated tissues from fresh-cut Chinese water-chestnuts (Eleocharis tuberosa). Sci Hortic (Amsterdam). 2022;297:110925. Available from: http://dx.doi.org/10.1016/j.scienta.2022.110925
- Bai M, Yao G, Ren Q, Li Q, Liu Q, Zhang Y, et al. Triterpenoid saponins and flavonoids from licorice residues with anti-inflammatory activity. Ind Crops Prod. 2018;125:50–8. Available from: https://doi.org/10.1016/j.indcrop.2018.08.075
- Srividhya M, Hridya H, Shanthi V, Ramanathan K. Bioactive amentoflavone isolated from Cassia fistula L. leaves exhibits therapeutic efficacy. 3 Biotech. 2017;7:33. Available from: https://doi.org/10.1007/s13205-017-0599-7
- Liu Y, Xia C, Chen B, Li X, Wu X, Ismail A, et al. Identification of selected flavonoids extracted from cap and stem of wild and cultivated Ganoderma species (Agaricomycetes) and bioactivities. Int J Med Mushrooms. 2025;27(6):61–79. Available from: https://doi.org/10.1615/intjmedmushrooms.2025058111
- Wang Z, Yang Q, Zhang H, He Y, Wang R, Lu X. Isolation, identification, and antibacterial activities of flavonoids from jujube (Ziziphus jujuba Mill.) fruit. Int J Fruit Sci. 2023;23(1):51–61. Available from: http://dx.doi.org/10.1080/15538362.2023.2186149
- Pasaribu A, Nurtjahja K. Isolation and characterization of flavonoid from leaves of Bauhinia kockiana Lour and antibacterial activity. J Phys Conf Ser. 2020;1542:012045. Available from: http://dx.doi.org/10.1088/1742-6596/1542/1/012045
- Liu Y, Yu X, Zhang W, Wang T, Jiang B, Tang H, et al. Prenylated chromones and flavonoids from Artocarpus heterophyllus with their potential antiproliferative and anti-inflammatory activities. Bioorg Chem. 2020;101:104030. Available from: https://doi.org/10.1016/j.bioorg.2020.104030
- Wang J, Zhou W, Huang X, Song S. Flavonoids with antioxidant and tyrosinase inhibitory activity from corn silk (Stigma maydis). Nat Prod Res. 2023;37(5):835–9. Available from: https://doi.org/10.1080/14786419.2022.2089986
- Akalin M, Karagoz S, Akyuz M. Application of response surface methodology to extract yields from stinging nettle under supercritical ethanol conditions. J Supercrit Fluids. 2013;84:164–72. Available from: http://dx.doi.org/10.1016/j.supflu.2013.10.004
- Toma M, Vinatoru M, Paniwnyk L, Mason TJ. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason Sonochem. 2001;8(2):137–42. Available from: https://doi.org/10.1016/s1350-4177(00)00033-x
- Sun H, Gao Y, An X, Jiang X, Piao X, Jin M, et al. Optimization of the culture medium of adventitious root culture to produce the flavonoids and the triterpenoids of Actinidia arguta by using an orthogonal design process. Plant Cell Tiss Organ Cult. 2021;144:545–54. Available from: https://link.springer.com/article/10.1007/s11240-020-01977-1
- Wu X, Tang Y, Sun Y, Xiang D, Wan Y, Wu Q, et al. Extraction of flavonoids and kinetics of purification by macroporous resins from quinoa. J Nanopart Res. 2020;22:181. Available from: https://ui.adsabs.harvard.edu/link_gateway/2020JNR....22..181X/doi:10.1007/s11051-020-04906-7
- Ding B, Wang Z, Yi R, Zhang S, Li X, She Z, et al. A modified QuEChERS method coupled with high resolution LC-Q-TOF-mass spectrometry for the extraction, identification and quantification of isoflavones in soybeans. Anal Methods. 2016;8:2259–66. Available from: https://doi.org/10.1039/C5AY03100A
- Cui L, Hou X, Li W, Leng Y, Zhang Y, Li X, et al. Dynamic changes of secondary metabolites and tyrosinase activity of Malus pumila flowers. BMC Chem. 2019;13:81. Available from: https://doi.org/10.1186/s13065-019-0602-y
- Zhou X, Wang J, Wang H, Lu P, Huang Q, Huang M, et al. Identification of extracted antioxidants from Citrus aurantium 'Changshanhuyou' residue against digestive enzyme activities and airway smooth muscle cells proliferation using UPLC-MS/MS. Food Biosci. 2024;61:104910. Available from: http://dx.doi.org/10.1016/j.fbio.2024.104910
- Tang H, Peng H, Wang F, Wang S, Yang L, Liu J, et al. Effects of combined administration of calcium, iron, zinc, chrysanthemum flavonoids, and DMSA on the treatment of lead intoxication in mice. J Biochem Mol Toxicol. 2019;34(2):e22425. Available from: https://doi.org/10.1002/jbt.22425
- Singh S, Tripathi A, Lal V, Singh D. High performance liquid chromatography analysis using rutin marker and estimation of phenolic and flavonoid compounds in the extracts of Indian medicinal plant Morus nigra L. Asian J Chem. 2019;31(8):1801-4. Available from: http://dx.doi.org/10.14233/ajchem.2019.22003
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
