Annals of Musculoskeletal Medicine
Femoral cartilage thickness in patients with systemic sclerosis: It’s relation to vitamin D
Gihan Omar1*, Rasha Ali Abdelmajeed1, Amal Hassan1, Yarah Aseem1 and Aliaa Hegazzy2
2Clinical Pathology Department, Minia University Hospital, Minia, Egypt
Cite this as
Omar G, Abdelmajeed RA, Hassan A, Aseem Y, Hegazzy A (2018) Femoral cartilage thickness in patients with systemic sclerosis: It’s relation to vitamin D. Ann Musculoskelet Med 2(1): 006-012. DOI: 10.17352/amm.000012Background: Systemic sclerosis (SSc) is a chronic autoimmune progressive connective tissue disease characterized by widespread vascular, immune and fibrotic changes of the skin and internal organs. Articular cartilage thickness previously investigated in few. Lower levels of vitamin D have been demonstrated in SSc patients and may be related to more sever disease of longer duration and extensive skin involvement. Aim of this study was to compare the levels of vitamin D and femoral cartilage thickness (FCT) in SSc patients with that of controls and to analyze the associations between the (FCT), vitamin D levels and SSc-disease parameters.
Material and Methods: Twenty five SSc patients diagnosed according to ACR/EULAR- 2013 classification criteria and twenty five controls; apparently healthy age and sex matched were studied. Serum levels of 25-hydroxyvitamin D (25[OH] D) were assessed by (ELISA), levels less than ≤ 10ng/mL are defined as deficiency, while > 10 ng/mL is defined as insufficiency. The thickness of femoral articular cartilage was measured by ultrasonography (MSU) in patients and controls. Three midpoint measurements were taken from each knee: lateral femoral condyle (LFC), femoral intercondylar area (ICA) and medial femoral condyle (MFC).
Result: We concluded that SSc patients seem to have thinner femoral cartilage values at all studied sites than that of control. A significant difference was found in measurement of right inter condylar area (p=0.029), right medial condyles (p=0.022), left inter condylar area (p=0.036), left lateral condyle and left medial condyle (p=0.001) between patients and control. Lower levels of vitamin D was significantly found in SSc patients than controls, predominantly among females (p= 0.009) with longer disease duration (p= 0.03) and sever skin involvement (p= 0.0001). Vitamin D was correlated with SSc severity scale as well as thinner femoral cartilage sites studied by MSU, while some sites shown correlation with disease activity parameters.
Introduction
Systemic sclerosis (SSc, scleroderma) is a complex connective tissue disease of unknown etiology characterized by multiorgan involvement, autoimmune heterogeneous clinical manifestations representing widespread vascular injury and progressive fibrosis of the skin and internal organs [1-3] .The incidence of SSc is about 20 cases per million populations per year and the prevalence is more than 250 patients per million populations in USA [4]. Major organ involvement leads to decreased survival in SSc, however, cardiopulmonary complications being the most frequent causes of disease related death. However, patients with SSc live longer and cardiac deaths are increasing [5]. In the last two decades, the survival of patients with systemic sclerosis (SSc) has significantly improved [6,7]. Nevertheless, SSc can still cause increased disability and reduced quality of life. Hand, tendon and joint involvement are other factors which can cause significant functional disability, leading to consequent disuse and worsening of bone loss in patients with SSc, contributing to a significant impairment in the quality of life of these patients [8-10].
American College of Rheumatology (ACR)/EULAR, 2013 developed a classification criteria assumed to improve sensitivity, which would lead to earlier diagnosis, and it also incorporates the autoantibodies that are commonly used for diagnostic purposes [11]. Vitamin D have been the focus of a growing number of studies in past years, demonstrating their function not only in calcium metabolism and bone formation, but also their interaction with the immune system since vitamin D receptors are expressed in different tissues. Numerous studies have been conducted to study whether vitamin D is associated with SSc; however, they produced varying results. [12-20]. Vitamin D deficiency has been documented in 80% of SSc patients. Low vitamin D levels in SSc patients are universal and independent of geographic origin or vitamin D supplementation [21,22]. Poor vitamin D status in SSc patients seems to be related to disseminated skin involvement and renal injury that may interfere with vitamin D synthesis. Also, Gastrointestinal involvement and malabsorption of dietary vitamin D in advanced intestinal disease are responsible for low levels of vitamin D [23,24]. Moreover, patients with SSc experience impairment in physical functioning and they are prone to a sedentary lifestyle and diminished sunlight exposure [25].
The association between SSc patients and vitamin D levels might also be explained by the hypothesis that low vitamin D level directly increases the risk of autoimmune disease. Vitamin D has been substantiated to have an antifibrotic effect on fibroblasts, inhibit synthesis of the extracellular matrix and regulate the skin’s immune system [26]. Furthermore; vitamin D can increase the activity of anti-inflammatory mediators and decrease the expression of proinflammatory cytokines [27]. Cartilage thinning may occur in Systemic Sclerosis. Vitamin D deficiency may change the balance of cartilage metabolism via reducing the synthesis of proteoglycan and/or increasing the metalloproteinase activity, leading to cartilage loss [28,29]. The serum human cartilage glycoprotein (HC gp-39), cartilage oligomeric matrix protein (COMP) and matrix metalloproteinase (MMP) levels were found to be elevated in patients with SSc [30]. We aimed to study levels of vitamin D in relation to the femoral cartilage thickness (FCT) in patients with SSc comparing that with controls that were matched for age and sex and to analyze the associations between the (FCT), vitamin D levels, SSc- disease activity and severity scores.
Materials and Methods
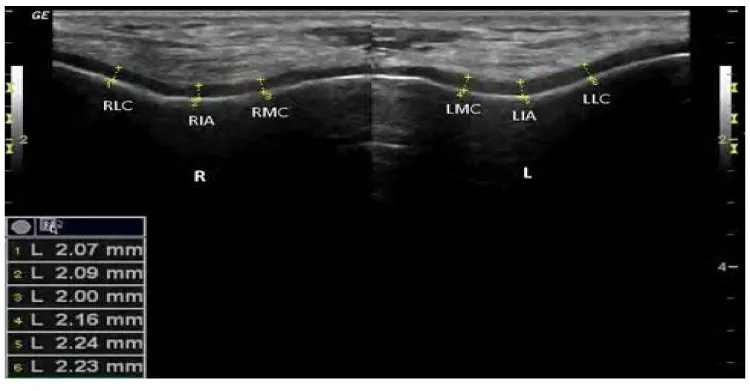
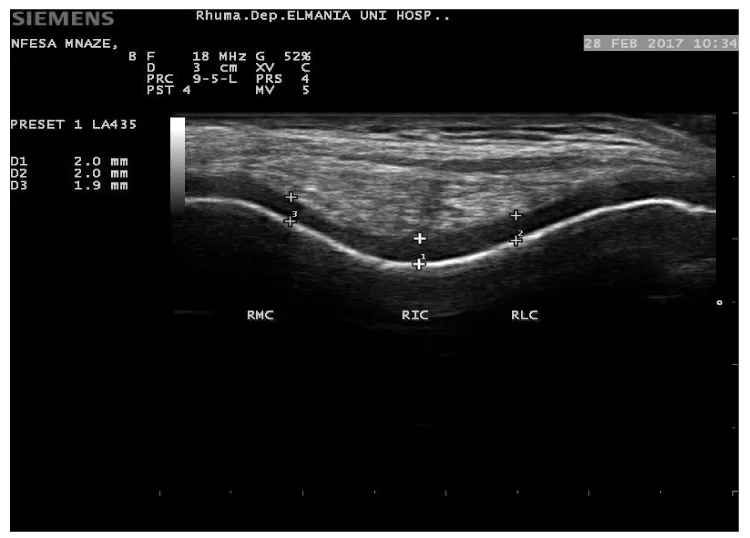
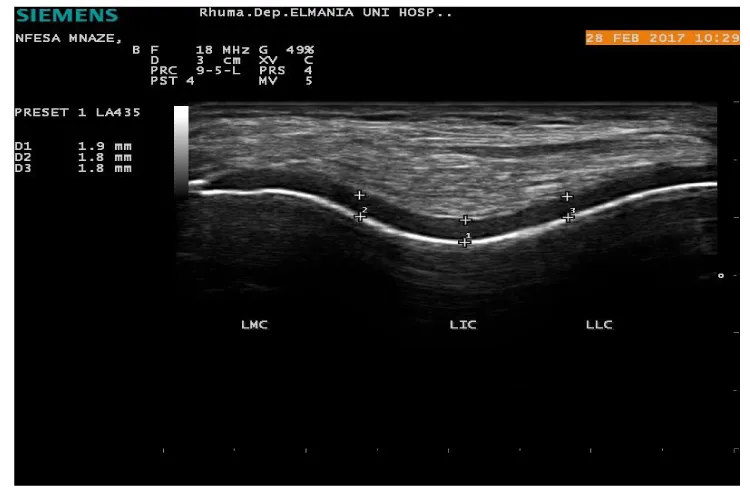
Twenty -five SSc patients (25) diagnosed according to the ACR/EULAR- 2013 classification criteria for SSc [11], were recruited from attendee of Rheumatology & Rehabilitation Department, Minia University hospital, Egypt; in the period of Nov., 2015 to August, 2016 and enrolled in this cross-sectional study. Excluded patients who known to have other connective tissue diseases overlap, juvenile scleroderma, localized subtype scleroderma and known metabolic diseases. Twenty-five (25); apparently healthy age and sex matched volunteers were included as controls from the same population. Patients’ oral and written consent was obtained and the study was approved by a local ethical committee. All subjects (patients and control) had a thorough history taking and clinical examination. Data concerning Vitamin D were reported {sun time exposure, area to be exposed to sun with approximate duration, skin tone and diet}. Assessment of disease severity was evaluated in Ssc patients according to Medsger Disease Severity Index (MDSI) [31], who defined severity as the total effect of the disease on organ function. Scales were developed from 0 (no documented involvement) to 4 (end stage disease) for each organ system: general (weight loss in kg), peripheral vascular (digital vascular ischemia), skin (mRSS), joint/tendon, muscle (weakness), gastrointestinal tract, lung, heart, and kidney. We considered a severe disease when the MDSI was superior to 3, according to previous studies. Modified Rodnan skin score (mRSS) was used to determine the extension of the skin involvement, classifying 17 anatomical sites from 0 (no skin involvement) to 3 (severe skin involvement), with maximal score of 51 [32]. Routine laboratory work investigations performed in SSc patients, including antinuclear antibody by ELISA, Calcium and phosphorus. Serum 25(OH) D biochemical assay using enzyme-linked immunosorbent assay (ELISA), were measured in all subjects (patients and controls). A serum 25(OH) D concentrations levels <30 and 10 ng/ml were defined as vitamin D insufficiency and vitamin D defciency, respectively [33,34], while levels >30 ng/ml were defined as vitamin D sufficiency [35] Musculoskeletal Ultrasound of both knees to assess femoral cartilage thickness in all subjects was performed using Siemens ACUSON P300 ultrasound system (Siemens, Healthcare, Boulevard, and Malvern, USA) portable machine with linear probe 10–18 MHz. Femoral cartilage was examined with patients in a supine position, and both knees were at maximum flexion. The probe was placed in the axial plane on the suprapatellar area, and the cartilage was visualized. Three midpoint measurements were taken from each knee: lateral femoral condyle (LFC), femoral intercondylar area (ICA) and medial femoral condyle (MFC) (Figure 1).
Statistical analysis
Analysis of data was completed using SPSS (Statistical program for social science) version 16. Data were expressed as mean±SD for parametric variables and as number and percent for non-parametric variable. Comparison between groups for parametric data was done by unpaired t-test and for non-parametric variables by Mann-Whitney U test. Chi–square (X2) test was used to compare qualitative variables. Spearman’s test was used for correlation of non-parametric variables.
Results
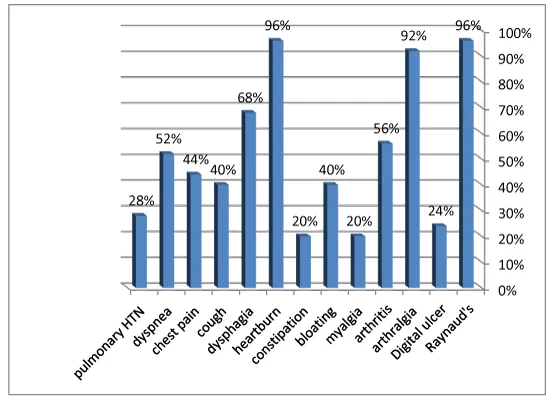
Of the twenty-five SSc patients enrolled, women were more prevalent in frequency, represented in 22(88%) and men in only 3(12%). Age was 33.3 ±8.1 years. Average disease duration was 5.6 ±4.3 years (Table 1). Range of Modified Rodnan skin score (mRSS) was 13-33 (23.2 ±5.8). Limited cutaneous (lcSSc) had a frequency of 18(72%) and diffuse cutaneous (dcSSc) frequency of 7 (28%). Clinical findings in SSc patients represented in (figure 2), 24 (96%) had Raynaud’s phenomenon, arthralgia in 23 (92%) and upper GIT symptoms, namely, dysphagia in 17 (68%). According to SSc disease severity scale, 5(20%) were mild, 13(52%) moderate and 7(28%) were active. Antinuclear antibody was positive in 18(72%) SSc patients.
The vitamin D levels range were 8.1-25.5 ng/ mL (13.2±5.2) and 10.5-25.9 ng/ mL (16.98±4.2) in SSc patients and control respectively. Insufficient 25(OH) D levels were found among controls >10 ng/mL, while SSc patients had deficient levels ≤10 ng/mL .Clinical significant differences between patients and controls vitamin D levels (p= 0.007) (Table 1). SSc patients seem to have thinner femoral cartilage values at all studied sites than that of control (Table 1). A significant difference was found in measurement of right inter condylar area (p=0.03), right medial condyles (p=0.02), left inter condylar area (p=0.04), left lateral condyle and left medial condyle (p=0.001) between patients and control (Figure 3).
We thought to further subdivide SSc patients, according to their serum vitamin D level into: Deficient serum vitamin D level ≤10 ng/ml, including 9 (36%) patients , range 8.1-10 ng/ mL (.96±.05) and insufficient vitamin D level >10 ng/ml, including 16 (64%) patients, range 10.9-25.5 ng/ mL (.96±.05) (Table 2). Average Modified Rodnan skin score (mRSS) was in deficient vitamin D level 26 ±3.8 {6 (66.7%) limited and diffuse in 3(33.3%)}, while in insufficient vitamin D level 21.6± 6.1 {12 (75%) limited and diffuse in 4 (25%)}. Patients with deficient vitamin D levels had poor sun time exposure than those with insufficient 25(OH) D levels (33.3% vs. 87.5%, respectively; p=0.04), moreover, they presented with thinner femoral cartilage values at all studied sites than that of insufficient vitamin D levels (Table 2). Vitamin D levels correlated with female sex (p= 0.009, r=0.514) and inversely correlated with disease duration (p= 0.04, r=-0.414), mRSS (p=0.0001, r= -0.667) and SSc disease severity scale (p=0.03, r=- 0.428).
Discussion
Deficient 25(OH) D serum concentrations were below 10 ng/ml in 36% and insufficient level >10 ng/ml in 64% of the studied SSc patients with no significant difference of limited versus diffuse types. Additionally, vitamin D inversely correlated with SSc- disease severity scales (p=0.03, r=- 0.428), Patients with high disease severity state and diffuse skin affection have low serum vitamin D level. Lower significant levels of vitamin D 25(OH) D levels were found among SSc patients than that of the controls (p= 0.007). Similarly, a meta-analysis study of Vitamin D levels in systemic sclerosis patients concluded that, SSc patients exhibited lower vitamin D levels compared with healthy controls. Vitamin D levels of diffused-type SSc patients were significantly lower than those in limited type SSc patients. Unlike us, the severity of clinical features was not associated with the extent of vitamin D deficit. They hypothesized that SSc patients, especially diffused type, have lower vitamin D levels and that the decrease of vitamin D levels might not be an accelerating factor of SSc severity [23].
In two previous studies for hypovitaminosis D at the same population, the first clinically studied hypovitamoniosis D in Sixty female patients complaining of LBP lasting more than 3 months. Hypovitaminosis D (25 OHD < 40 ng/ml) was found in 49/60 patients (81%) and 12/20 (60%) of controls, with an odds ratio of 2.97. They postulated although many risk factors related to sun exposure, clothing, diet, and pregnancy were significantly correlated with vitamin D levels in patients, only limited duration of sun exposure, contributing 55% to the variance of 25 OHD, limited areas of skin exposed (13%), and increased number of pregnancies (2%), were significant determinants of vitamin D levels in those patients. It was concluded that, despite the sunny climate, hypovitaminosis D is prevalent among Egyptian women, the major determinant of hypovitaminosis D in their patients was limited sun exposure [36].
The second study had the same conclusion that, Hypovitaminosis D is prevalent in the Egyptian females 61.5%. The prevalence and determinants of hypovitaminosis D among Two hundred females aged from 17–76 years with a mean± SD of (38.96± 13.7). Among the 200 females, 123 female (61.5%) had hypovitaminosis D (defined as 25- OH vit D <40 ng/ml), while 77 female (38.5%) had desirable vit D levels (25- OH vit D >40 ng/ml). Both PTH (p< 0.001) levels and ALP (p< 0.001) were significantly higher in females with hypovitaminosis D than those with desirable levels. Sun exposure duration, areas of skin exposed, and gravidity were the main determinants of vit D levels, despite being in a sunny environment and rich available dietary resources. Community education and awareness were encouraged regarding dietary requirement of Vitamin D, food rich with, specifically according to age group, even food fortification was recommended. Sun exposure duration, timing of the day and minimal area to be exposed, effect of repeated pregnancies as determinants of vitamin D level, should be also considered [37].
Our results demonstrated an unfavorable impact of vitamin D deficiency on femoral cartilage thickness at all measured sites by ultrasonography. Low levels of vitamin D thought to change the balance of cartilage metabolism via reducing the synthesis of proteoglycan and or increasing the metalloproteinase activity, leading to cartilage loss [29]. Healthy bone and cartilage turnover depends on adequate availability of vitamin D [38]. In concordance with our study, Arnson and colleagues implied that female patients with low levels of vitamin D (less than 10 ng/ml) have thinner femoral cartilage than those with vitamin D more than 10 ng/mL and SSc patients have a high prevalence of vitamin D deficiency [21]. Hypovitaminosis D in SSc patients can be attributed to skin involvement and renal injury that might interfere with vitamin D synthesis and malabsorption due to advanced intestinal disease. Carmel, et al. reported that SSc patients have higher levels of IgM anti-25(OH) D antibodies, although anti-vitamin D was detected among both SSc patients and healthy controls. They raise questions about a possible additional immunogenic role of both vitamin D metabolites. Their observation of vitamin D antibodies, which are more frequent in SSc patients, thought to aid in differentiating scleroderma patients from healthy controls [39]. Vitamin D antibodies may also cause vitamin D deficiency in other autoimmune diseases. Carvalho and team found vitamin D antibodies in a subset of patients with SLE, they reported association between anti-vitamin D antibodies and anti-dsDNA in SLE patients, suggesting its role in SLE pathogenesis [40]. Handono, similarly postulated that low vitamin D levels in patients with SLE may be caused by anti-vitamin D antibodies [41]. The deficiency of 25(OH) D3 had a direct relationship with increase disease activity and nephritis in Egyptian SLE patients, suggesting the need for vitamin D supplementation in these patients [42]. Femoral cartilage thickness values evaluated by ultrasonography in 29 SLE patients study, found to be increased in those patients who were using corticosteroids [43].
Our results in SSc presented here are in concert with that of Kilic and colleagues, who compared the femoral cartilage thickness (FCT) in female SSc patients with that of controls who were matched for age, body mass index and osteoarthritis prevalence. Forty female patients with SSc and 85 female controls were included. The thickness of femoral articular cartilage was measured by ultrasonography in patients and controls. They concluded that Patients with SSc had thinner femoral cartilage compared with controls. However, at that study, vitamin D was not evaluated in patients or postulated for its role in either the diseases or cartilage thickness [30].
Conclusion
Our present study emphasize on the relation between hypovitaminosis D and cartilage thickness in SSc, relatively small no of patients and lack of cartilage metabolism biomarkers assessment represents a limitation in our research. Factors determining vitamin D in diet and sunlight exposure needed to further detailed, it seemed having pivotal role in our patients. Further clarifications are needed for vitamin D correlation with SSc, and whether vitamin D is a cause, accelerating factor or consequence of SSc. Again, we still stick on our previous recommendations to have patient education regarding sun light exposure and quality of diet, rich in vitamin D, perhaps food fortification and supplementation are advised.
- Jimenez SA (2013) Role of endothelial to mesenchymal transition in the pathogenesis of the vascular alterations in systemic sclerosis. ISRN Rheumatol 23. Link: https://goo.gl/bDTa1D
- Wollheim FA (2005) Classification of systemic sclerosis. Visions and reality. Rheumatology 44: 1212-1216. Link: https://goo.gl/D6Kd5A
- Pattanaik D, Brown M, Bradley C, Postlethwaite AE (2015) Pathogenesis of Systemic Sclerosis. Front Immunol 6: 272. Link: https://goo.gl/5K9UcE
- Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH (2010) Rheumatology. 5th ed. Philadelphia, PA: Mosby.
- Komócsi A, Vorobcsuk A, Faludi R, Pintér T, Lenkey Z, et al. (2012) The impact of cardiopulmonary manifestations on the mortality of SSc: a systematic review and meta-analysis of observational studies. Rheumatology 51: 1027-1036. Link: https://goo.gl/eeiQHU
- Tyndall AJ, Bannert B, Vonk M, Airò P, Cozzi F, et al. ( 2010) Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 69: 1809-1815. Link: https://goo.gl/N36c9k
- Sampaio-Barros PD, Bortoluzzo AB, Marangoni RG, Rocha LF, Del Rio AP et al. (2012) Survival, causes of death, and prognostic factors in systemic sclerosis: analysis of 947 Brazilian patients. J Rheumatol 39: 1971-1978. Link: https://goo.gl/hVoj4t
- Nguyen C, Ranque B, Baubet T, Bérezné A, Mestre-Stanislas C, et al. (2014) Clinical, functional and health-related quality of life correlates of clinically significant symptoms of anxiety and depression in patients with systemic sclerosis: a cross-sectional survey. Link: https://goo.gl/jkZjZx
- Mouthon L (2013) Hand involvement in systemic sclerosis 42: 1616-1626. Link: https://goo.gl/2Yx1CH
- Bassel M, Hudson M, Baron M, Taillefer SS, Mouthon L, et al. ( 2012) Physical and occupational therapy referral and use among systemic sclerosis patients with impaired hand function: results from a Canadian national survey. Clin Exp Rheumatol 30: 574-577. Link: https://goo.gl/xPXRxa
- van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, et al. (2013) classifcation criteria for systemic sclerosis: an American College of Rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum 65: 2737-2747.Link: https://goo.gl/4yDVaS
- Dankers W, Colin EM, van Hamburg JP, Lubberts E ( 2017) Vitamin D in autoimmunity: molecular mechanisms and therapeutic potential. Front Immunol 7: 697. Link: https://goo.gl/aGHcGn
- Denton CP (2016) Advances in pathogenesis and treatment of systemic sclerosis 16: 55-60. Link: https://goo.gl/hFXf9c
- Giuggioli D, Colaci M, Cassone G, P. Fallahi,F. Lumetti et al. ( 2017). Serum 25-OH vitamin D levels in systemic sclerosis: analysis of 140 patients and review of the literature. Clin Rheumatol 36: 583-590. Link: https://goo.gl/UVMk46
- Montabone E, Data V, Carignola R ( 2016) Vitamin D status and quality of life in systemic sclerosis patients. J Clin Rheumatol 22: 229-230. Link: https://goo.gl/arFVMq
- Bivona G, Agnello L, Pivetti A, Salvatore M, Concetta S, et al. (2016) A Association between hypovitaminosis D and systemic sclerosis: true or fake? Clin Chim Acta.; 458:115-119. Link: https://goo.gl/PBKQpD
- Zhang L, Duan Y, Zhang TP, Xiao-Lei Huang, Bao-Zhu Li, et al. (2017) Association between the serum level of vitamin D and systemic sclerosis in a Chinese population: a case control study 20: 1002-1008. Link: https://goo.gl/xA8D1W
- Corrado A, Colia R, Mele A, Valeria Di B, Antonello T (2015) Relationship between body mass composition, bone mineral density, skin fibrosis and 25(OH) vitamin D serum levels in systemic sclerosis. Link: https://goo.gl/U2bjaC
- Caramaschi P, Dalla Gassa A, Ruzzenente O, Alessandro V, Viviana R, et al. (2010) Very low levels of vitamin D in systemic sclerosis patients. Clin Rheumatol 29: 1419-1425. Link: https://goo.gl/g9JaZm
- Groseanu L, Bojinca V, Gudu T, Saulescu L, Predeteanu D.et al. ( 2016)Low vitamin D status in systemic sclerosis and the impact on disease phenotype. Eur J Rheumatol 3: 50-55. Link: https://goo.gl/UUK3du
- Arnson Y, Amital H, Agmon-Levin N, Alon D, Sa´nchez-Castaño´n M, et al. ( 2011) Serum 25-OH vitamin D concentrations are linked with various clinical aspects in patients with systemic sclerosis: a retrospective cohort study and review of the literature. Autoimmun Rev 10: 490-494. Link: https://goo.gl/9BMoA6
- Vacca A, Cormier C, Mathieu A, Kahan A, Allanore Y ( 2011 ) Vitamin D levels and potential impact in systemic sclerosis. Clin Exp Rheumatol 29: 1024-1031. Link: https://goo.gl/EEUyGC
- Lin An, Ming-hui Sun, Feng Chen, Jin-ran Li (2017) Vitamin D levels in systemic sclerosis patients: a meta-analysis Drug Des Devel Ther 11: 3119-3125. Link: https://goo.gl/wtt8du
- Calzolari G, Data V, Carignola R, Angeli A ( 2009) Hypovitaminosis D in systemic sclerosis. J Rheumatol 36: 2844-2845. Link: https://goo.gl/8vRFva
- Belloli L, Ughi N, Marasini B (2010) Vitamin D in systemic sclerosis. Clin Rheumatol 30: 145-146. Link: https://goo.gl/q8FoA1
- Yang CY, Leung PS, Adamopoulos IE, Gershwin ME (2013) The implication of vitamin D and autoimmunity: a comprehensive review. Clin Rev Allergy Immunol 45: 217-226. Link: https://goo.gl/GeryPK
- Fernandes de Abreu DA, Eyles D, Féron F (2009) Vitamin D, a neuroimmunomodulator: implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology 34: 265-277. Link: https://goo.gl/FvKYuL
- Al-Jarallah KF, Shehab D, Al-Awadhi A, Nahar I, Haider MZ, et al. (2012) Are 25(OH) D levels related to the severity of knee osteoarthritis and function? 21: 74-78. Link: https://goo.gl/W76W89
- Malas FU, Kara M, Aktekin L, Ersöz M, Ozçakar L (2013) does vitamin D affect femoral cartilage thickness? An ultrasonographic study. Clin Rheumatol.33: 1331-1334. Link: https://goo.gl/rn2Qqx
- Kilic G, Kilic E, Akgul O, Ozgocmen S (2014 ) Decreased femoral cartilage thickness in patients with systemic sclerosis 347:382-386. Link: https://goo.gl/iSxiP1
- Medsger TA Jr, Silman AJ, Steen VD, Black CM, Akesson A, et al. (1999) A disease severity scale for systemic sclerosis: development and testing. J Rheumatol 26: 2159-2167.Link: https://goo.gl/QQpz7e
- Furst DE, Clements PJ, Steen VD, Medsger TA Jr, Masi AT, et al. (1998) The modified Rodnan skin score is an accurate reflection of skin biopsy thickness in systemic sclerosis. J Rheumatol 25: 84-88. Link: https://goo.gl/oQWjEm
- Hollis BW, Wagner CL (2005) Normal serum vitamin D levels [letter] 352: 515-516. Link: https://goo.gl/kSfJA7
- Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, et al. (2005) Estimates of optimal vitamin D status. Osteoporosis Int 16: 713-716. Link: https://goo.gl/Sk4F67
- Scharla S, Chapuy M, Schattauer (1996) V: Determination of 25-vitamin D in human serum and plasma. Exp Clin Endocrinol Diabetes 104: 289-292. Link:
- Lotfi A, Abdel-Nasser AM, Hamdy A, Omran AA, El-Rehany MA. (2007) Hypovitaminosis D in female patients with chronic low back pain. Clin Rheumatol 26: 1895-1901. Link: https://goo.gl/E5CUuK
- Gihan MO, Ahmad L, Jehan ABDEL-WAHHAB, Essmat A EL-SHARKAWY ( 2010) Hypovitaminosis D among females of Minia Governorate, Egypt .International Journal of Rheumatic Diseases 13: 172-177.Link: https://goo.gl/v7LEMs
- Felson DT, Niu J, Clancy M, Aliabadi P, Sack B, et al. (2007) Low levels of vitamin D and worsening of knee osteoarthritis: results of two longitudinal studies. Arthritis Rheum 56: 129-136. Link: https://goo.gl/oQ9XTn
- Carmel NN, Rotman-Pikielny P, Lavrov A, Levy Y (2015) Vitamin D Antibodies in Systemic Sclerosis Patients: Findings and Clinical Correlations 17: 80-84.Link: https://goo.gl/fwjsPj
- Carvalho JF, Blank M, Kiss E, Tarr T, Amital H, et al. (2007) Anti-vitamin D, vitamin D in SLE: preliminary results 1109: 550-557 Link: https://goo.gl/27V2Po
- Handono K (2013) Vitamin D Serum level and disease activity in patients with systemic lupus erythematosus 2: 35-40. Link: https://goo.gl/jr5PTk
- Abdel Galil SM, El-Shafey AM, Abdul-Maksoud RS, El-Boshy M (2017) Interferon alpha gene expression and serum level association with low vitamin D levels in Egyptian female patients with systemic lupus erythematosus 27: 199-209. Link: https://goo.gl/mxM77R
- Kaya A, Kara M, Tiftik T, Tezcan ME, Öztürk MA, et al. (2012) Ultrasonographic evaluation of the femoral cartilage thickness in patients with systemic lupus erythematosus. Rheumatol Int 33: 899-901. Link: https://goo.gl/AHdCX6
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
