Annals of Musculoskeletal Medicine
Subjects with substituted hypothyroidism oxidize more lipids and carbohydrates during exercise
Jean-Frederic Brun1*, Stephanie Metrat1, Jean-Marie Nguyen1, Marlene Richou1, Fatiha M’Rabta1, Orianne Villard1, Francois Bughin1, Christine Fedou1, Ariane Sultan2, Antoine Avignon2, Jacques Mercier2, and Eric Raynaud De Mauverger1
2Service de de Nutrition, Lapeyronie Hospital CHRU Montpellier, France
Cite this as
Brun JF, Metrat S, Nguyen JM, Richou M, Rabta FM, et al. (2018) Subjects with substituted hypothyroidism oxidize more lipids and carbohydrates during exercise. Ann Musculoskelet Med 2(1): 013-016. DOI: 10.17352/amm.000013Subjects with hypothyroidism substituted with levothyroxine (HS) are known to be poor responders to weight reducing strategies. Since muscle energy metabolism is regulated by thyroid hormones we compared the oxidation of fat and carbohydrates (CHO) during exercise in HS versus controls. We compared 52 patients (48 women, 4 men, age 49 t years, levothyroxine dose 25-250 micrograms / day) to a control group of 2081 patients matched for sex, age, BMI and percentage of fat during an exercise calorimetry with 4 submaximal 6 minutes steps. At the same power intensity HS on the average oxidize more fat (p = 0.009) and use more oxygen (p = 0.00019). Lipid oxidation culminates at the same power intensity(39,4±2,4 vs 38,6±0,3 watts) but its maximal oxidation rate is significantly higher in the HS group (10.32±0.47 vs. 9, 06±0.10 mg / min / kg muscle p = 0.02) and carbohydrate oxidation during the final level (1866.6 ± 77.3 vs1705.45 ± 11.5 mg / min p = 0.029 ). The maximal lipid oxidation rate is correlated with the dose of levothyroxine (r = 0.331, p <0.05).HS patients exhibit an overall increase of energy expenditure during exercise, oxidizing more lipids at mild to moderate intensities and more CHO at high intensities. This latter mechanism could result into an orexigenic effect of physical activity contributing to resistance to weight loss.
Introduction
Obesity is currently considered as an epidemic condition in many countries, with serious health implications [1]. A potential association between thyroid function and overweight control is discussed in the recent literature [2]. In a recent review [3], it can be read that even rather small differences in thyroid function within a population are associated with differences in body mass index and thus with the prevalence of obesity. However, the mechanism of this association is rather complex [2,3]. An important aspect of this question is the very common complaint of patients treated by levothyroxine for thyroid disease who gain too much weight and have most difficulty to lose this weight with conventional approaches [2].
Since the energy metabolism of muscle is regulated by thyroid hormones [3], we hypothesized that a shift in the balance of substrates oxidized during exercise might be the explanation of the difficulty in initiating or maintaining weight loss of individuals suffering from hypothyroidism and substituted with levothyroxine. Thyroid hormones are known to regulate muscle cell phenotype and to induce important alterations in mitochondrial functions [4,5]. It could be thus hypothesized that they induce a decrease in the ability to oxidize lipids and a parallel rise in carbohydrate use as a fuel for muscle contraction. However, very little is known about this issue.
Therefore, we compared the oxidation of the energy substrates during exercise in subjects in HS versus controls.
Subjects and methods
Subjects: We compared 52 patients (48 women, 4 men, age 49 years, levothyroxine dose 25-250 micrograms / day) to a control group of 2081 patients from our database of 4500 subjects, carefully matched for sex, age, BMI and percentage of fat (Table 1).
Bioelectrical impedance measurements: Prior to the exercise-test, subjects’ body composition was assessed with bioimpedance analysis with a six terminal impedance plethismograph BIACORPUS RX 4000 Biacorpus RX4000, (Soagil, 8 avenue Jean-Jaurès 92130 Issy-les-Moulineaux, France) with data analysis with the software Body Comp 8.4. This device measures total resistance of the body to an alternative electric current of 50 kHz [6,7].
Exercise Calorimetry: Each subject underwent an exercise calorimetry, in the morning after an overnight fast. The test [8-10], is performed on an ergometric bicycle connected to an analyzer allowing the analysis of the gaseous exchange cycles by cycle. EKG monitoring and measurements of VO2, VCO2, and respiratory exchange ratio (RER) are performed during the test. After a period of 3 minutes at rest, and another period of initial warm-up at 20% of the predicted maximal power (PMP) for 3 minutes, the 6-min workloads set at approximately 30, 40, 50 and 60% of PMP are performed. The phase of recovery comprises two periods during which a monitoring of respiratory and cardiac parameters is maintained: active recovery at 20% of the PMP during 1 minute; passive recovery (ie, rest) during the 2 following minutes. At the end of each stage, during the fifth and sixth minutes, values of VO2 and VCO2 are recorded. These values are used the calculation of the respective rates of oxidation of carbohydrates and
= 4.585 VCO2 – 3.2255 VO2 (1)
Lipid Oxidation (mg/min) = -1. lipids by applying the classical stoichiometric equations of indirect calorimetry:
Carbohydrates (mg/min) 7012 VCO2 + 1.6946 VO2 (2)
These calculations are performed on values of the 5-6th minutes of each step, since at this CO2 production from bicarbonate buffers compensating for the production of lactic acid becomes negligible. The increment in carbohydrate oxidation above basal values appears to be roughly a linear function of the developed power and the slope of this relation is calculated, providing the glucidic cost of the watt. The increase in lipid oxidation adopts the shape of a bell-shaped curve: after a peak, lipid oxidation decreases at the highest power intensities.
The exact mechanism of this reduction in the use of the lipids at the highest power intensities is actually imperfectly known: a reduction in lipolysis is likely to explain a part of it, together with a shift of metabolic pathways within the muscle fiber. The empirical formula of indirect calorimetry that gives the lipid oxidation rate is, as reminded above:
Lipid oxidation (mg/min) = -1.7 VCO2 + 1.7 VO2 (3)
It is easy to deduce from this formula that the relation between power (P) and oxidation of lipids (Lox) displays a bell-shaped curve of the form:
Lox = A.P (1-RER) (4)
The smoothing of this curve enables us to calculate the power intensity at which lipid oxidation becomes maximal, which is the point where the derivative of this curve becomes equal to zero. Therefore the LIPOXmax calculation is only application of the classical empirical equation of lipid oxidation used in calorimetry.
The maximal lipid oxidation point (LIPOXmax in mg.kg-1.min-1): it is the exercise intensity at which lipid oxidation reaches its maximal level before decreasing while carbohydrate utilization further increases. It is calculated after smoothing of the curve plotting lipid oxidation as a function of power.
Theoretical VO2max is calculated according to Wasserman’s formula. VO2max ACSM is calculated from the linear relationship between VO2 and heart rate as the value of VO2 which correspond to the theoretical maximal heart rate
Statistics
Curve comparisons were performed with two way analysis of variance with the software « Sigmastat 3.5 » from Jandel Scientific.
Results
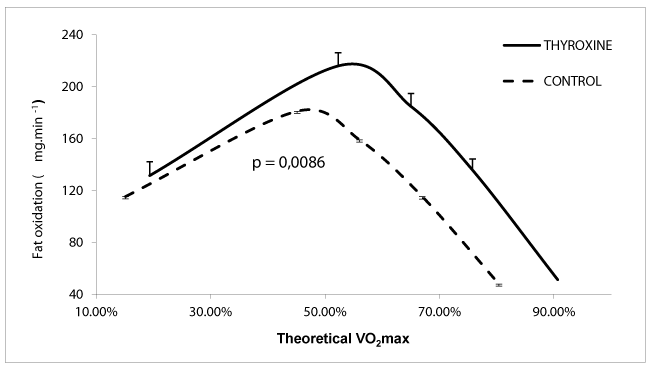
As shown on figure 1, lipid oxidation peaked at the same level (39.4 ±2.4 vs 38.6 ± 0.3 watts) but its maximum flow rate was significantly higher in the group of substituted hypothyroidism (10.32 ± 0.47 vs. 9,06±0,10 mg / min / kg muscle p = 0.02). For the same power intensity, HSs oxidize on the average more lipids (p = 0.009) and consume more oxygen (p = 0.00019).
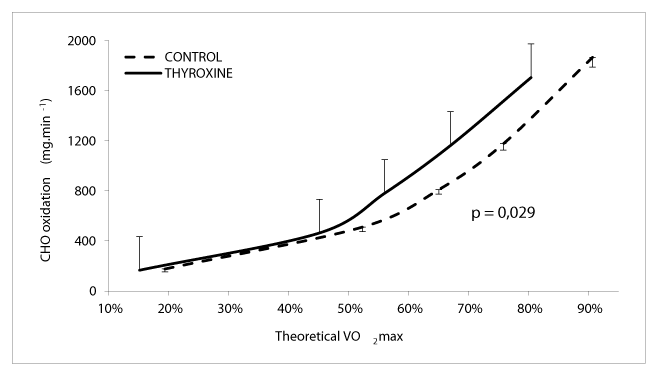
As shown on figure 2, carbohydrate oxidation at the last level (60% VO2max) is higher in patients compared to controls (1866.6 ± 77.3 vs 1705.45 ± 11.5 mg / min p = 0.029).
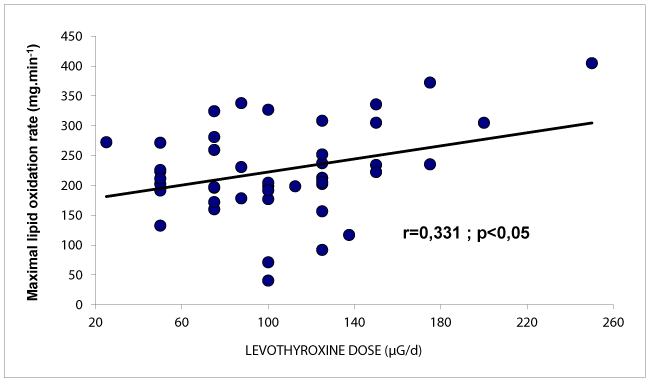
Figure 3 shows that there is a positive correlation between the dose of T4 and the maximal rate of lipid oxidation during exercise. By contrast the dose of levothyroxine was not correlated to CHO oxidation (r=0.16 NS).
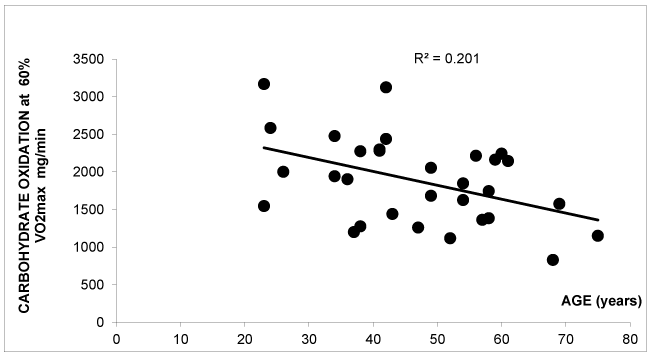
Figure 4 shows that there is a negative correlation between CHO oxidation at 60% VO2max and age in the group of patients (r=-0.448 p=0.0009) but not in controls (r=-0,171 NS). There is no correlation between age and maximal fat oxidation (r=0.046 NS) in patients, but this correlation is found in controls (r=-0.171 p<0.0001). Due to the design of the study an effect of gender cannot be studied.
Discussion
As reminded in the introduction, individuals suffering from hypothyroidism and substituted with levothyroxine are known to have more difficulty for initiating or maintaining weight loss. This study shows that these patients exhibit an increase in exercise energy metabolism, oxidizing more lipids in light to moderate power intensities and more carbohydrates at high power intensities. Even more, we evidence a positive correlation between the dose of thyroxin and the maximal rate of lipid oxidation during exercise.
Therefore, our initial working hypothesis that these patients may oxidize less fat at exercise, explaining their difficulties to lose weight, was not confirmed. The results show a quite different picture: actually, being treated by T4 enhances both lipid and carbohydrate oxidation, and the more the patients take T4 the more they burn fat at exercise.
This finding is in accordance with current physiological literature, that shows a stimulating effect of thyroid hormones on both fat and carbohydrate oxidation [2-4].
An alternative explanation for the difficulty to loss weight in these patients thus arises. Exercise performed at intensities higher that those where lipid oxidation predominates may result in exaggerated breakdown of carbohydrate stores, resulting in glycogen depletion and thus an orexigenic effect of physical activity contributing to overeating and weight gain, as described in obesity [11]. While a low volume of exercise targeted at the level of lipid oxidation is able to induce a weight loss prolonged for at least 3 years [12], a low volume of exercise targeted at higher intensities often makes the opposite and induces weight gain [11].
The main strength of this cross-sectional study is that we had the opportunity to use a huge database of exercise calorimetries to build a very large (more than 2000 subjects) well matched control group, that provided us enough power to detect rather subtle differences. By contrast one can say that those differences are not very marked and require such a large sample to be evidenced.
On the whole this study shows for the first time that patients treated for a thyroid disease by levothyroxine do not exhibit any defect in substrate oxidation during exercise. On the opposite they have an excellent ability to burn fat at exercise, even higher on the average than the one of matched controls. Theoretically, with such a fair capacity of lipid oxidation, they should be expected to respond to exercise training procedures targeted on lipid oxidation. This kind of training is efficient on the long term (more than 36 months) and its efficacy is proportional to the ability to oxidize fat at exercise [12]. However, there is another striking characteristic of these patients during exercise: they exhibit a slightly higher carbohydrate oxidation rate, which may promote glycogenic depletion, overeating and paradoxical weight gain [11]. We hypothesize that this property can contribute to the resistance to slimming procedures observed in these patients [2,3].
Surprisingly, as shown on figure 4, this ability to oxidize more carbohydrates at high intensity exercise is mostly found in young subjects and vanishes with age. This relationship was unexpected. A look at figure 4, can lead to think that this property is due to the sex hormone status, and that this effect is found in young women but not in postmenopausal ones. Such a decrease in the ability to oxidize CHO at exercise when patients get more than 50 years old is not found in the matched control group. In another study we previously reported in 50 years old men an age-related decline in the use of CHO as an energy source during exercise at all intensity that can be corrected by exercise training and is thus likely to be a consequence of sedentarity [13]. We suggest that this can also be an explanation for this finding in women treated by thyroid hormones, but this explanation remains speculative. By contrast we find an increased O2 consumption at high intensity (which explains why calorimetry calculations evidence a higher CHO oxidation rate) and this exaggerated O2 consumption may result from increased mitochondrial function, and possibly with some degree of oxidation uncoupling, due to thyroid hormones. Therefore, those patients well-treated by thyroid hormones may at high Intensity exercise be in a situation mimicking mild hyperthyroidism. This hypothesis requires to be tested.
All this suggests that exercise more tightly targeted on lipid oxidation can be efficient to control weight in these patients, while high intensities would have the opposite effect. Actually, there is no reported follow-up study of thyroid hormone-treated patients addressing this issue. This hypothesis is currently being investigated in our unit.
- Caballero B (2007) The global epidemic of obesity: an overview. Epidemiol Rev 29: 1–5. Link: https://tinyurl.com/y849hod8
- Biondi B (2010) Thyroid and obesity: an intriguing relationship. J Clin Endocrinol Metab 95: 3614–3617. Link: https://tinyurl.com/ydxdjmgw
- Laurberg P, Knudsen N, Andersen S, Carle A, Pedersen IB, et al. (2012) Thyroid Function and Obesity. European Thyroid Journal 1:159-167. Link: https://tinyurl.com/y7m826eo
- dos Santos RA, Giannocco G, Nunes MT (2001) Thyroid hormone stimulates myoglobin expression in soleus and extensorum digitalis longus muscles of rats: Concomitant alterations in the activities of Krebs cycle oxidative enzymes. Thyroid 11: 545–550. Link: https://tinyurl.com/yamvf3u4
- Bahi L, Garnier A, Fortin D, Serrurier B, Veksler V, et al. (2005) Differential effects of thyroid hormones on energy metabolism of rat slow- and fast-twitch muscles. J. Cell. Physiol 203: 589–598. Link: https://tinyurl.com/y7uknaeb
- Brun JF, Guiraudou M, Mardemootoo C, Traore A, Raingeard I, et al. (2013) Validation de la mesure segmentaire de la composition corporelle en comparaison avec la DEXA: interêt de la mesure de la masse grasse tronculaire. Sci Sports 28: 158-162. Link: https://tinyurl.com/ydam492n
- Guiraudou M, Maimoun L, Dumas J-M, Julia M, Raingeard I, et al. (2015) Composition corporelle mesuree par impedancemetrie segmentaire (BIAS) et performance de sprint chez les rugbymen / Body composition measured by bioimpedance segmental (BIAS) analysis and sprint performance in rugby players. Sci Sports 30: 298-302. Link: https://tinyurl.com/yc56743e
- Brun JF, Romain AJ, Mercier J (2011) Maximal lipid oxidation during exercise (Lipoxmax): from physiological measurements to clinical applications. Facts and uncertainties Sci Sports 26: 57-71. Link: https://tinyurl.com/y7v22f7j
- Brun JF, Malatesta D, Sartorio A (2012) Maximal lipid oxidation during exercise: A target for individualizing endurance training in obesity and diabetes? J Endocrinol Invest 35: 686-691. Link: https://tinyurl.com/y9lzr7hg
- Perez-Martin A, Dumortier M, Raynaud E, Brun JF, Fedou C, et al. (2001) Balance of substrate oxidation during submaximal exercise in lean and obese people. Diabetes Metab 27: 466-474. Link: https://tinyurl.com/yabf2sb6
- Brun JF, Romain AJ, Sferlazza A, Fedou C, Raynaud de Mauverger E, et al. (2016) Which individuals become fatter when they practice exercise? Sci Sports 31: 214- 218. Link: https://tinyurl.com/ydz62ulp
- Drapier E, Cherif A, Richou M, Bughin F, Fedou C, et al. (2018) Long term (3 years) weight loss after low intensity endurance training targeted at the level of maximal muscular lipid oxidation. Integr Obesity Diabetes 4: 1-6. Link: https://tinyurl.com/yalygwu5
- Manetta J, Brun JF, Prefaut C, Mercier J (2005) Substrate oxidation during exercise at moderate and hard intensity in middle-aged and young athletes vs sedentary men. Metabolism 54:1411-1419. Link: https://tinyurl.com/y79ugnak
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
