Annals of Musculoskeletal Medicine
Expanding the phenotype of spastic paraplegia 26: Report of 4 cases with hearing dysfunction
Carolina Lopes1,3*, Fernando Silveira1, Goreti Nadais1 and Miguel Leão2,3
2MD, Neurogenetics Unit, Department of Medical Genetics, University Hospital Center of Sao Joao, Porto, Portugal
3MD, Faculty of Medicine, University of Porto, Porto, Portugal
Cite this as
Lopes C, Silveira F, Nadais G, Leão M (2019) Expanding the phenotype of spastic paraplegia 26: Report of 4 cases with hearing dysfunction. Ann Musculoskelet Med 3(2): 014-017. DOI: 10.17352/amm.000018Background: Spastic Paraplegia 26 (SPG26) is a complex type of spastic paraplegia caused by B4GALNT1 gene pathogenic variants, and is characterized by childhood/adolescence onset of progressive spastic paraplegia associated with mild to moderate cognitive impairment and developmental delay, dysarthria, cerebellar ataxia, and peripheral neuropathy.
Results: We report four additional cases, from three Portuguese families, of SPG26, demonstrating high phenotypic heterogeneity, both inter-familial and intra-familial. Using neurophysiological studies, we describe hearing dysfunction as a feature of SPG26.
Conclusions: SPG26 is rare and familiarity with the typical presentation may be helpful to the diagnosis and allow an increased awareness of this disorder.
Introduction
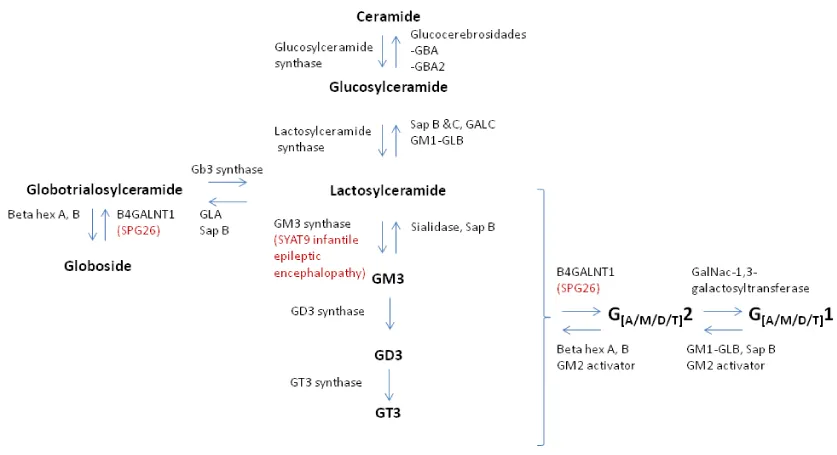
Autosomal Recessive (AR) spastic paraplegia 26 (SPG26) is a complex type of Hereditary Spastic Paraplegia (HSP) characterized by childhood/adolescence onset of progressive spastic paraplegia, mild/moderate cognitive impairment, developmental delay, dysarthria, cerebellar ataxia and polyneuropathy [1-3]. SPG26 is caused by B4GALNT1 (12q13.3) pathogenic variants, encoding beta-1,4 N-acetylgalactosaminyltransferase 1 (GM2/GD2 synthase), an enzyme involved in biosynthesis of complex gangliosides [4].
We report four additional cases, from three Portuguese families, of complicated HSP revealing B4GALNT1 pathogenic variants. We describe hearing dysfunction as a feature of SPG26, not previously described in this condition.
Materials and Methods
Patient registries were consecutively assessed. Data on clinical, imagiologic and neurophysiological studies was analysed.
Results and Discussion
Patient-1
25-year-old male presented with psychomotor retardation and gait disturbance. His delivery was full-term; his perinatal period and childhood were uneventful. His parents, non-consanguineous, and a 17-year-old brother were healthy. His 12-year-old brother had psychomotor retardation (Patient-2). A maternal aunt had intellectual disability and tip-toe gait from 25 years old.
He started walking at 2 years old and was clumsy. At 12 years old his gait further deteriorated, with tip-toe walking, and muscle atrophy in lower limbs was noted.
Physical examination revealed mildly asymmetric, predominantly distal, spastic paraparesis, global hyperreflexia, bilateral Babinski sign and foot drop, with spastic and steppage gait. Bilateral stocking pinprick and vibration sensitive deficits were present. Intelligence quotient was 44.
Laboratory investigations and neuroaxis MRI were normal. Nerve Conduction Studies (NCS) revealed sensitive and motor axonal polyneuropathy. Auditory Evoked Potentials (AEP) showed very low amplitude and poorly defined waves I to V, bilaterally. Audiogram was normal.
Patient’s karyotype was 46,XY. Array Comparative Genomic Hybridization (aCGH), genetic testing for FMR1 and multigene panel for AR HSP were normal. Clinical exome study revealed B4GALNT1 homozygotic pathogenic variant c.682C>T (p.Arg228*).
Patient-2
12 year-old male was referred for psychomotor retardation. His delivery was full-term and his neonatal period was uneventful. He walked alone at 2 years old, and was clumsy. Intelligence quotient was 54.
Physical examination was remarkable for global hyperreflexia and bilateral dysdiadochokinesia.
Laboratory investigations, NCS and neuroaxis MRI were unremarkable. AEP revealed a bilaterally low V/I amplitude ratio. Audiogram was normal.
aCGH was unremarkable. Considering his brother´s genetic test results, targeted analysis of B4GALNT1 revealed the same homozygotic pathogenic variant.
Patient-3
45-year-old male, born from healthy and non-consanguineous parents, presented with progressive tetraparesis. First symptoms were noted at 15 years old and he was unable to walk by his thirties. The disease later progressed with dysartrodysphonia and dysphagia.
Psychomotor development was normal until five years old, when he was admitted for meningitis. Thenceforth, intellectual impairment was noted.
Neurological examination revealed bilateral sensorineural hearing loss, hypotonic tetraparesis, global hyporeflexia, bilateral Babinski sign and pes cavus.
Laboratory investigations and neuroaxis MRI were normal. AEP revealed severe sensorineural hearing loss on the right hear and moderate on the left. NCS were compatible with sensitive and motor axonal polyneuropathy.
Patient’s karyotype was 46,XY. A multigene panel for hereditary neuropathies was normal. Clinical exome study revealed the same B4GALNT1 homozygotic pathogenic variant present in Patient-1 and Patient-2. Apparently, no inbreeding relationships between both families exist.
Patient-4
7-year-old female presented with gait difficulty and psychomotor retardation. She was born at 38 weeks and the neonatal period was unremarkable. Her parents, non-consanguineous, and her 10-year-old brother were healthy. She sat alone at 10 months and started walking at 18 months.
Physical examination revealed symmetrical, predominantly distal, spastic paraparesis, hyperreflexia in legs, bilateral Babinski sign and foot drop, with spastic and steppage gait. Bilateral glove and stocking vibration sensitive deficit was present, as well as pes cavus. Right dysmetria was noted in finger-to-nose test.
Laboratory investigations, brain MRI and NCS were normal. AEP revealed normal wave I latency, I-III interval mildly prolonged and V/I amplitude ratio in the lower limit of normal.
Patient’s karyotype was 46,XX. aCGH and genetic testing for FMR1 were normal. Clinical exome study revealed B4GALNT1 homozygotic pathogenic variant c.395del (p.(Pro132GInfs*7)).
Conclusion
Farag, et al., reported a consanguineous Kuwaiti family in which 5 siblings had complicated HSP [5]. In a follow-up study of this family, Wilkinson, et al., identified a putative 22.8-cM disease locus on chromosome 12p11.1-q1 [6]. By exome sequencing of 5 families with AR HSP, Boukhris, et al., identified 5 different B4GALNT1 homozygous pathogenic variants and described the associated phenotype [2].
Our cases presented features already described in SPG26 (Table 1). All patients revealed intellectual impairment; Patient-1, Patient-3 and Patient-4 presented spastic paraparesis; Patient-2 did not present motor deficits but had global hyperreflexia. Cerebellar involvement was noted in Patient-2 and Patient-4. Polyneuropathy was present in Patient-1 and Patient-3.
These cases confirm the high inter-familial and intra-familial phenotypic heterogeneity in SPG26 (Table 1), remarkably evident in Patient-1 and Patient-2, from the same family, although the absence of spastic paraparesis and polyneuropathy can be explained by the younger age of Patient-2.
All patients had neurophysiological evidence of auditory pathway impairment. In Patient-3, meningitis sequelae may contribute to hearing impairment, although an alternative explanation for other patients’ results could not be identified. Previous reports described AEP studies in patients with SPG [7-11]. Pedersen and Trojaborg [8], concluded that one of 13 patients studied had abnormal results to visual, auditory and somatosensory stimulation. Sawhney, et al., [9], demonstrated that AEP were altered in 13 of 25 patients with SPG. Tedeschi, et al., [10], concluded that AEP and other brainstem evoked potentials changes, were more common in complicated than in pure forms of SPG. All the aforementioned studies report patients without genetic characterization and the diagnosis was made in clinical basis alone. Manganelli, et al., [11] demonstrated AEPs changes in families with SPG5 molecular diagnosis. Hearing impairment, which may eventually worsen with age, is, to the best of the authors’ knowledge, a feature not previously described in patients with SPG26 associated with B4GALNT1 pathogenic variants. Only Patient-3 reported hypoacusia, and these neurophysiological changes have no clinical repercussion in the remaining patients, which is according to previous reports [8,10], demonstrating AEP changes in SPG patients without auditory complaints [8,10].
B4GALNT1 encodes b-1,4 N-acetyl-galactosaminyl transferase 1, which catalyzes the transfer of N-acetyl-galactosamine into GM3, GD3 and globotriaosylceramide by a b-1,4 linkage [2]. GM3 synthase, encoded by ST3GAL5, mediates the sialylation of lactosylceramide to form GM3, the root structure for all downstream a- and b-series gangliosides [12] (Figure 1). Yoshikava, et al., showed that in eight children homozygous for ST3GAL5 c.694C>T pathogenic variant, their auditory function was characterized by absence of middle ear muscle reflexes, distortion product otoacoustic emissions and cochlear microphonics, as well as abnormal auditory brainstem responses and cortical AEP [13].
B4GALNT1 homozygotic pathogenic variant found in the first three cases had already been described and demonstrated as pathogenic in a Brazilian family with SPG26 [2]. Considering the rarity of SPG26, the absence of consanguinity between the family of Patient-1 and Patient-2 and the family of the Patient-3, and the history of the discovery and Portuguese colonization of Brazil, the authors hypothesize that this can be explained by a founder effect originating from the Portuguese population.
The pathogenic variant found in Patient-4 had already been described and demonstrated as pathogenic in a Spanish family with SPG26 [2].
Next generation sequencing is revolutionizing our understanding in medical genetics, allowing the identification of the molecular basis of several disorders, not otherwise detected. Understanding the clinical manifestations and the phenotypic heterogeneity of SPG26 will help elucidate the pathogenesis and mechanisms of neurodegeneration and to establish correlations between the genotype and the phenotype in this disease.
- Harding AE (1983) Classification of the hereditary ataxias and paraplegias. Lancet 1: 1151-1155. Link: http://bit.ly/35yI8D3
- Boukhris A, Schulle R, Loureiro J, Lourenço CM, Mundwiller E, et al. (2013) Alteration of Ganglioside Biosynthesis Responsible for Complex Hereditary Spastic Paraplegia. Am J Med Genet 93: 118-123. Link: http://bit.ly/2M1G4f5
- Harlalka GV, Lehman A, Chioza B, Baple EL, Maroofian R, et al. (2013) Mutations in B4GALNT1 (GM2 synthase) underlie a new disorder of ganglioside biosynthesis. Brain 136: 3618-3624. Link: http://bit.ly/2Pwm9Hk
- Xu YH, Barnes S, Sun Y, Grabowski GA (2010) Multi-system disorders of glycosphingolipid and ganglioside metabolism. J Lipid Res 51: 1643-1675. Link: http://bit.ly/2sDsGH4
- Farag TI, El-Badramany MH, Al-Sharkawy S (1994) Troyer syndrome: report of the first 'non-Amish' sibship and review. Am J Med Genet 53: 383-385. Link: http://bit.ly/2s1NMPx
- Wilkinson PA, Simpson MA, Bastaki L, Patel H, Reed JA, et al. (2005) A new locus for autosomal recessive complicated hereditary spastic paraplegia (SPG26) maps to chromosome 12p11.1-12q14. J Med Genet 42: 80-82. Link: http://bit.ly/2Z1idRZ
- Yoshikawa M, Go S, Takasaki K, Kakazu Y, Ohashi M, et al. (2009)Mice lacking ganglioside GM3 synthase exhibit complete hearing loss due to selective degeneration of the organ of Corti. Proc Nat Acad Sci 106: 9483-9488. Link: http://bit.ly/36J8GBJ
- Pedersen L, Trojaborg W (1981) Visual, auditory and somatosensory pathway involvement in hereditary cerebellar ataxia, friedreich's ataxia and familial spastic paraplegia. Electroencephalogr Clin Neurophysiol 52: 283-297. Link: http://bit.ly/2tuMPQ9
- Sawhney LMS, Bansai SK, Upadhyay PK, Chopra JS (1993) Evoked potentials in hereditary spastic paraplegia. ItaL J Neurol Sci 14: 425-428. Link: http://bit.ly/35ArvXv
- Tedeschi G, Allocca S, Di Costanzo A, Carlomagno S, Merla F, et al. (1991) Multisystem involvement of the central nervous system in Strümpell's disease. A neurophysiological and neuropsychological study. J Neurol Sci 103: 55-60. Link: http://bit.ly/34zVRrI
- Manganelli F, Pisciotta C, Dubbioso R, Iodice R, Criscuolo C, et al. (2011) Electrophysiological characterization in hereditary spastic paraplegia type 5. Clin Neurophysiol 122: 819-822. Link: http://bit.ly/2EuD86E
- Inokuchi IJ, Go S, Yoshikawa M, Strauss K (2017) Gangliosides and hearing. Biochim Biophys Acta Biomembr 1861: 2485-2493. Link: http://bit.ly/2Z01i1Z
- Yoshikawa M, Go S, Suzuki S, Suzuki A, Katori Y, et al. (2015) Ganglioside GM3 is essential for the structural integrity and function of cochlear hair cells. Hum Mol Genet 24: 2796-2807. Link: http://bit.ly/2Z27qGZ
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
