Annals of Musculoskeletal Medicine
Intravenous single dose of tranexamic acid safely reduces blood loss and the need for transfusion in elderly patients with hip fracture. A randomized double-blinded controlled trial at 1-year follow-up
Francisco A Miralles-Muñoz1*, Rosario Martin-Grandes1, Daniel Martinez-Mendez1, Gerard Mahiques-Segura1, Alejandro Lizaur-Utrilla1,2 and María Flores Vizcaya-Moreno3
2Department of Traumatology and Orthopaedics, Faculty of Medicine, Miguel Hernández University, N-332 km 87s/n, 03550 San Juan de Alicante, Alicante, Spain
3Unit of Nursing Research, Faculty of Health Sciences, Alicante University, Ctra San Vicente del Raspeig s/n, 03690 San Vicente del Raspeig, Alicante, Spain
Cite this as
Miralles-Muñoz FA, Martin-Grandes R, Martinez-Mendez D, Mahiques-Segura G, Lizaur-Utrilla A, et al. (2023) Intravenous single dose of tranexamic acid safely reduces blood loss and the need for transfusion in elderly patients with hip fracture. A randomized double-blinded controlled trial at 1-year follow-up. Ann Musculoskelet Med 7(2): 009-015. DOI: 10.17352/amm.000032Copyright Licence
© 2023 Miralles-Muñoz FA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: A hip fracture usually presents significant blood loss in the perioperative period, with a transfusion rate of 20-60%. In order to reduce the complications associated with this procedure, the administration of Tranexamic Acid (TXA) has been implemented in the treatment of perioperative anemia. The objectives were to evaluate the effectiveness and safety of a single dose of intravenous Tranexamic Acid (TXA) in reducing perioperative blood loss and the requirement for transfusion in elderly patients undergoing hip fracture surgery within one postoperative year.
Methods: A double-blind randomized controlled trial was conducted on 129 patients with hip fractures. After randomization, at the start of the surgery, 65 patients received a single dose of 1 gram of intravenous TXA (TXA group), and 64 received a placebo (placebo group). The primary effectiveness outcomes were the total blood loss and transfusion rate. The primary safety outcome was the rate of thromboembolic events. Data on surgical or medical infection, readmission and death were also collected.
Results: The TXA group had a significant decrease in blood loss (p = 0.006) and requirement for transfusion (p < 0.001) compared with the placebo group. Likewise, there were no thromboembolic events in the TXA group and seven in the placebo group (p = 0.006). Mortality within 1-year postoperatively was not significantly different (p = 0.115).
Conclusion: Using a single dose of intravenous TXA at the start of the surgery significantly reduces blood loss and the requirement for transfusion without increasing the risk of thromboembolic events or mortality within 1-year postoperatively in patients with hip fracture undergoing surgery.
Registration number: NCT03211286. https://clinicaltrials.gov/ct2/show/NCT03211286.
Level of evidence: I.
Introduction
Perioperative anaemia requiring blood transfusion in elderly patients with hip fractures is a severe health problem. Recently, several randomized studies [1,2] have reported the efficacy and safety of Tranexamic Acid (TXA) in reducing blood loss in patients with hip fractures who underwent surgery, with a follow-up between four postoperative days and three months [1-4] and only one randomised study had 6-month follow-up [5]. The risk of Venous Thromboembolism (VTE) after hip fracture surgery is usually described until 3 postoperative months, with the highest risk during the immediate postoperative period (one to six weeks) [6]. Nevertheless, some authors have demonstrated that hip fracture patients can have a risk of VTE up to one postoperative year [7,8]. Thus, the safety of routine use of TXA in hip fracture patients in the longer-term remains inconclusive.
The purposes of this study were to evaluate the effectiveness of a single dose of intravenous TXA in reducing perioperative blood loss and requirement for transfusion in elderly patients undergoing hip fracture surgery, and to assess the safety of TXA within one postoperative year. The hypothesis was that administering a single dose of intravenous TXA would decrease the perioperative bleeding and reduce the need for transfusion without increased thrombotic risk and mortality within one postoperative year.
Methods
This single-centre, randomised, placebo controlled, double blinded trial was approved by the institutional review board (PI2018-141) and included in a public registry (ClinicalTrials.gov NCT03211286). Informed consent was obtained prior to randomisation. This research was performed under the Declaration of Helsinki International Ethical Guidelines, and the protocol was conducted and reported according to the Consolidated Standards of Reporting Trials (CONSORT).
Consecutive patients with hip fractures admitted to our institution from January 2019 to September 2020 were eligible for the study. The inclusion criteria were age over 65, a hip fracture that occurred within 24 hours prior to admission, and surgical treatment within 48 hours after admission. The exclusion criteria were: 1) Groups IV-V of the American Society of Anesthesiologists (ASA) scale 2) tumoral pathologic fracture; 3) concomitant fracture; 4) refusal to receive blood products; 5) antiaggregant platelet treatment in the three days before surgery; and 6) described contraindications for TXA [8]. During the pandemic of the SARS-CoV-2 virus, positive patients were excluded because venous thrombosis was a potential complication in those patients [9].
Randomization
Randomization was based on a computer-generated number list using the block method by an independent assistant. Each assignment was sealed in a consecutively numbered opaque envelope opened by the nurse who prepared the intravenous solutions in the operating room. Patients were allocated into one of two groups: 1) TXA group, whose patients received 1 gram of intravenous TXA (Amchafibrin, Rottapharm Madaus, Germany) diluted in 100 ml of saline solution; 2) Placebo group, whose patients received an equivalent volume of intravenous saline solution. Masking was ensured by preparing the same volume of solution with an identical appearance. Just before the surgical incision, both treatments were performed. The surgeons, anesthetists, and patients were blinded to the assignment until the completion of the study. The dose of intravenous TXA chosen for this study was based on previous reports [10,11].
Surgical procedure
All surgical procedures were performed under spinal anesthesia. Extracapsular fractures were treated with a trochanteric femoral nail or dynamic hip screw, and intracapsular fractures with a bipolar hemiarthroplasty (usually in patients of age over 75 years) or total hip arthroplasty patients (patients under that age), acting under widely admitted indications. Diathermy was routinely used. At the end of the operation, a deep vacuum drain was placed for 24 hours.
With the proposal of minimising the effect of isovolumetric hemodilution, postoperative fluid therapy was standardized for the first 24 hours with 1500 ml of saline solution, unless the patient needed other treatment. In a standardized manner, all patients received antibiotic prophylaxis with first-generation cephalosporins for 24 hours, and thromboembolic prophylaxis with low-molecular-weight heparin for 30 days, according to the protocols of our center. All patients were postoperatively mobilized under the assistance of a physiotherapist on the first postoperative day.
Evaluation and outcome measures
A standardized protocol for co-management between orthopaedic surgeons and geriatricians was used from admission to discharge. Comorbidity patient was categorized using the ASA scale [12] and Charlson index [13]. Patient evaluation was made preoperatively and at one, three, six, and 12 postoperative months, unless death had occurred before. If the patient does not return for examination, telephone contact with patients or their relatives is performed. Two independent surgeons who were blinded to the study groups evaluated all outcomes.
The primary effectiveness outcomes were the total blood loss and transfusion rate. Patients were monitored with serial measurements of Haemoglobin (Hb) during the stay. Patients received a transfusion of packed red cells if their Hb fell below 8 g/dl, or less than 9 g/dl if they had symptomatic anemia or heart disease. The total blood loss was calculated according to widely accepted mathematical formulas based on the Hb levels and the estimated blood volume [14,15]. For this purpose, final Hb was the lowest level within four postoperative days [15].
To calculate the patient’s blood volume (BV, measured in liters), different formulas were used according to the gender of the patient:
For men = (0,3669 x H3) + (0,03219 x W) + 0,6041
For women = (0,3561 x H3) + (0,03308 x W) + 0,1833
[H, height (meters). W, weight (kilograms)]
To calculate the hemoglobin lost with bleeding (Hbloss), the following formula was applied:
Hbloss = BV x (Hbi – Hbe) x 0.001 + Hbt
[BV, the estimated volume of body blood (lt). Hbi, the hemoglobin of the patient before surgery (g/dl). Hbe, the lowest point of hemoglobin during admission (g/dl). Hbt, is the total amount of allogeneic Hb transfused. A unit of banked blood was considered to contain 52 g Hb, according to measurements at our hospital’s blood centre].
And finally, to calculate the estimated blood loss (EBL), the following formula was used:
EBL (ml) = 1000 x Hbloss/Hbi
The primary safety outcome was the rate of thromboembolic events. Patients were clinically monitored for these events until one postoperative year. If there was suspicion of any event, the diagnosis was confirmed by doppler ultrasonography for Deep Venous Thrombosis (DVT), computed tomography scan for pulmonary thromboembolism, magnetic resonance imaging for cerebral stroke, and Electrocardiogram (ECG) and troponin level for myocardial infarction. Data on surgical or medical infection, readmission and death were also collected.
Statistical analysis
According to a previous study on TXA for hip fracture surgery, a reduction in blood loss of 500 ml was considered clinically relevant [1]. For a power of 80% and a two-sided type I error of 5%, 60 patients per group were needed. Assuming a drop-out rate of 5%, at least 63 patients per group were required.
Statistical analysis was performed using SPSS software v. 19 (SPSS Inc, Chicago, USA). The Kolmogorov-Smirnov test was used to examine the normal distribution of continuous data. Analyses between groups were performed with the chi-square, Fisher’s exact, or nonparametric Mantel-Haenszel test for categorical variables, and the t-Student test or nonparametric Mann Whitney U-test for continuous variables. The paired t-Student test or Wilcoxon signed-rank test was used to compare preoperative and postoperative data. Multivariate logistic regression analyses were planned to identify independent risk factors for main outcome variables, including in the model only the variables with a univariate p - value < 0.1. Data were shown as Odds Ratio (OR) with 95% Confidence Interval (CI). The goodness of fit of the logistic regression model was analysed using the Hosmer-Lemeshow test. Statistical significance was considered for p values less than 0.05 in all tests.
Results
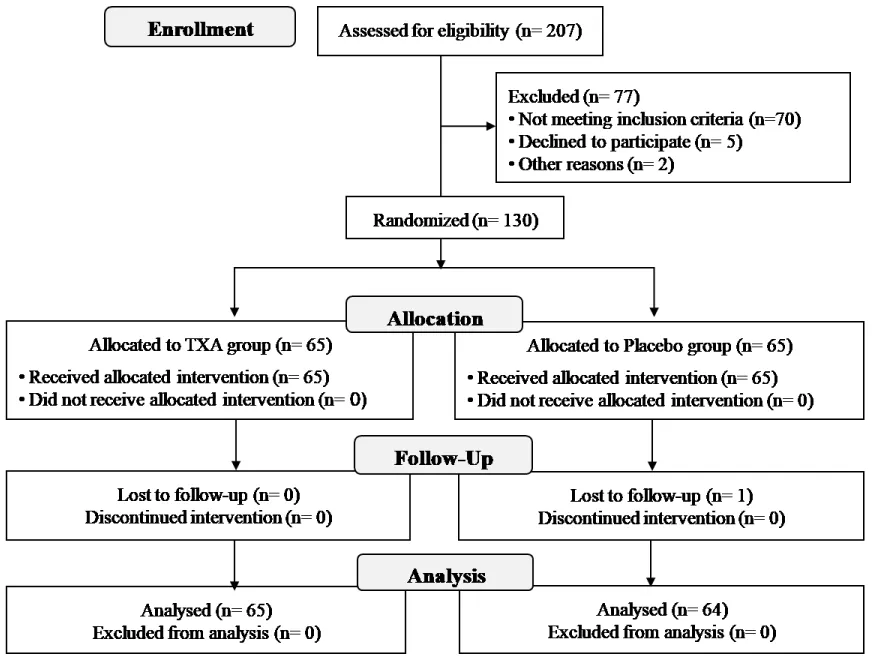
From January to December 2020, 207 patients with a hip fracture undergoing surgery were assessed for eligibility. Seventy-seven patients were excluded for various reasons, including failure to meet inclusion criteria (70 patients), decline to participate (five), or language barrier (two). The remaining 130 patients were randomised into the TXA group (65 patients) or control group (65 patients). One patient in the control group was then excluded due to follow-up loss (Figure 1). Thus, 129 patients were analyzed, 76% were females with a mean age of 81.8 years (range 69 to 100). The baseline data of both groups are shown in Table 1. There were no significant differences between groups, specifically in mean Hb level on admission (p = 0.226) or patient blood volume (p = 0.845). In the analysis by subgroups according to the fracture type, there were no significant differences in the baseline characteristics, except that intracapsular fractures presented longer surgery time (p = 0.019). Postoperative follow-up was one year in all patients.
Blood loss and transfusion
The mean total blood loss during the entire admission (Table 2) was significantly lower in the TXA group (962.3 ml, SD 511.7) compared with the placebo group (1328.0 ml, SD 752.4) (p = 0.006). There were five (7.7%) patients requiring transfusion in the TXA group and 22 (34.4%) in the placebo group (p < 0.001) (Table 2). Overall, patients with extracapsular fractures had a higher mean blood loss (1207.4 ml, SD 701.2) than those with intracapsular fractures (958.7 ml, SD 515.3) but without statistical significance (p = 0.052). There was no significant difference between extracapsular and intracapsular fractures in transfusion rate (p = 0.169). For the risk of blood transfusion, multivariate analysis adjusted for potential factors (Table 3) revealed that only the TXA treatment (OR, 0.14; 95% CI, 0.04 to 0.4; p = 0.001) and Hb level on admission (OR, 0.52; 95% CI, 0.3 to 0.7; p = 0.001) were significant predictors. Thus, TXA was a protective factor for transfusion, and inversely the placebo treatment was shown to be a risk factor (OR, 7.04; 95% CI, 2.2 to 22.4; p = 0.001). The Hosmer-Lemeshow test determined a good fit for the data from the logistic regression model (p = 0.984).
Safety outcomes
There were seven thromboembolic events within one year postoperatively, all in the placebo group (p = 0.006). Three patients were diagnosed with symptomatic DVT at 20, 89 and 91 postoperative days, three others had a cerebral stroke at 43, 44 and 236 postoperative days, and one patient suffered a myocardial infarction 35 days after surgery. There was no case of pulmonary embolism. Concerning the fracture type, there were no significant differences in thromboembolic event rate (p = 0.612). Multivariate analysis for the risk of thromboembolic events was not performed due to the low rate. The postoperative infection rate was 4.6% (three patients) in the TXA group compared with 7.8% (five patients) in the placebo group (p = 0.350). All those patients required early revision surgery. There was no significant relationship between infection and surgery time (p = 0.437) or blood transfusion (p = 0.529). Except for deceased patients, there were no cases of readmission.
The cumulative mortality (Table 2) was not significantly different between groups at 30 days (p = 0.653) or 90 days (p = 0.509). At one year, four (6.1%) patients in the TXA group and nine (14.0%) in the placebo group had died, but this difference was not significant (p = 0.115). Only postoperative infection (p = 0.034) showed a significant association with 1-year mortality in the univariate analysis. No other covariate presented a p - value less than 0.1, so the multivariate analysis was rejected. In the subgroup analysis according to the fracture type, there was no significant difference between extracapsular and intracapsular fractures in thromboembolic event rate (p = 0.612) or 1-year mortality (p = 0.113).
Discussion
The main finding of this study was that the administration of 1 g of intravenous TXA at the start of the surgery significantly reduced total blood loss and transfusion rate. The use of TXA was not associated with an increased risk of thromboembolic events or 1-year mortality. There was a high rate of excluded patients, although it is well known that hip fractures in the elderly have a certain rate of loss of follow-up and mortality.
Significant reduction of blood loss and need for transfusion were also reported by most randomised studies that used a single dose [2,4,16], two doses [1,17,18], or three doses [5] of intravenous TXA. Conversely, Zufferey, et al. found no significant differences in reducing blood loss or transfusion between the TXA and placebo groups, even though they used two doses, one at skin incision, and another three hours later [19].
Given the heterogeneity of the intravenous doses used in the different studies, a comparison is difficult. In the only study randomising one dose, two doses and placebo, the authors found no significant difference between one-dose and two-dose groups in blood loss, but the transfusion rate was significantly lower in the two-dose group [20]. Nevertheless, analysing the combined data from randomised studies, the mean reduction of blood loss using one-dose of TXA compared to the placebo group was 30%, and the decrease in the transfusion rate was 55% [2,4,11,16]. With two doses of TXA these data were 32% and 52%, respectively [1,3,17,18,21], and with three doses were 33% and 57%, respectively [5]. The above data suggested that the effectiveness of intravenous TXA was similar regardless of the number of doses. Recent metaanalyses also found that the frequency and dosage of intravenous TXA did not influence its beneficial effect [22,23]. Compared to the topical administration of TXA, several metanalyses reported that intravenous TXA was more effective in decreasing the need for transfusion [22,24].
As in the present study, Nikolaou, et al. found that preoperative Hb level and TXA were the only significant predictor of transfusion [2]. In agreement with other authors, total blood loss was higher in extracapsular fractures than intracapsular fractures, although the difference was not statistically significant [25,26]. Nikolaou, et al. reported significant differences in blood loss and transfusion rates between patients with extracapsular fractures who received intravenous TXA and those who received a placebo, but these differences were not significant in the patients with intracapsular fractures [2]. In the present study, extracapsular fractures presented greater blood losses and allogenic blood transfusions compared to intracapsular fractures, although not reaching statistical significance as a risk factor for transfusion.
Regarding the safety of the TXA treatment, conflicting results have been reported. Zufferey, et al. reported nine (16%) thromboembolic events with two doses of intravenous TXA according to the patient weight, given at skin incision and three hours later, and three (6%) in the placebo group, and although that difference was not statistically significant, the authors did not recommend TXA for hip fracture surgery [19].
Conversely, in another randomised study administering TXA pre- and post-operatively, Tengberg, et al. found no thromboembolic events in the TXA group [1]. However, regarding 90-day mortality, they did find a higher rate in the TXA group (27%) compared to the placebo treatment (10%), but although this difference seems important, it was not statistically significant and the influence of TXA on excess mortality could not be determined [1]. In addition, that study was underpowered because the inclusion of patients was stopped for administrative reasons unrelated to the results.
Contrary to those outcomes, all the remaining randomised studies with a follow-up of at least three months reported similar rates of a thromboembolic event, complication, and 90-day mortality between TXA and placebo groups using two [17,18] or one [4] doses of intravenous TXA. Using two doses, Zhang, et al. reported two (3%) thromboembolic events in the TXA group and one (2%) DVT in the placebo group, and the 90-day mortality rate was 1.6% and 3.3%, respectively [17]. Watts, et al. also with two doses, reported a thrombotic events rate of 7% in the TXA group and 9% in the placebo group with no significant difference between them, and the 90-day mortality rate was 14% in the TXA group, and 16% in the placebo group [18]. In the only randomised study with a follow-up of six months, Chen, et al. used three doses of intravenous TXA, and they found an incidence of thromboembolic events of 16% in the TXA group and 14% in the placebo group, while the 6-month mortality was 6% in the TXA group and 3.5% in the placebo group, with no significant differences between groups [5].
With one dose of intravenous TXA as in the present study, Ma, et al. reported 14% of DVT in the TXA group, and 13% in the placebo group, while no other thrombotic events were observed after a follow-up of three postoperative months [4]. Zhou, et al. also using one dose and a follow-up of one month, found three (3%) thromboembolic events in the TXA group, and seven (7%) in the placebo group, although that difference was not significant [18]. Similar findings were found in other randomised studies that used only one dose of intravenous TXA, although their follow-ups were between 48 hours and four days [2,11]. In the present study, there were three DVT and three strokes in the placebo group, while in most randomised studies DVT represented the most frequent vascular complication, regardless of assigned treatment.
Several recent metaanalyses reported that the short follow-up of the published trials limited the drawing of definitive conclusions on the TXA safety for hip fractures [24,27]. To our knowledge, the present study has the most extended follow-up to date evaluating potential adverse events of TXA for hip fracture surgery. Despite the fact that the mean duration of the effect of IV TXA is 3 hours, we considered establishing a 1-year follow-up to analyze mortality in patients with hip fractures who, in addition to the risk inherent to their traumatic process and subsequent surgery, could be associated with complications derived from the use of TXA (thromboembolic events) that could influence the mortality rate [7,8]. Other studies also performed a 1-year follow-up, but in cardiac surgery [28,29].
Nevertheless, the study also had several limitations. Due to safety considerations, high-risk patients were excluded from the study. Thus, the results may not be generalizable, and the safety of TXA in those patients remains unproven. In the analysis of subgroups according to the fracture type, there was a significant difference in surgical time, which could compromise the homogeneity of the samples. However, we believe that this does not influence the effectiveness or safety of the administration of intravenous TXA. The study involved multiple surgical procedures and was dominated by internal fixation procedures, increasing the potential for bias. There is no standard reference for recording perioperative blood loss. For the primary effectiveness outcome, the mathematical calculation of the blood loss based on clinical measurements proposed by Good, et al. was used [15]. Like others, this method is not validated and maybe a source of error with a tendency to overestimate blood loss. However, it was applied to compare two groups, with a standardized postoperative fluid therapy used to minimize the effect of isovolumetric hemodilution. The Good’s method is usually used in randomised studies of TXA [1,2,20] and it is considered the most reliable [30]. Regarding the safety outcome, subclinical or asymptomatic DVT might have gone undetected. On the other hand, the sample size was based on blood loss. Given the low frequency of thromboembolic events, the study may be underpowered to detect differences in these complications. Furthermore, the optimal timing and dosage of intravenous TXA are still unclear, but the administration of a standardized single dose used in the present study has shown its effectiveness in other randomised studies [4,16] and similar to those that used more than one dose [17,18].
Conclusion
The use of a single dose of intravenous TXA at the start of the surgery reduces blood loss and requirement for transfusion significantly without increasing the risk of thromboembolic events or mortality within one year postoperatively in patients older than 65 years with hip fractures undergoing surgery.
Ethics approval: This study was performed in line with the principles of the Declaration of Helsinki.
The Institutional Review Board approved this randomized study. Ethical Committee of Clinical Investigation, Elda University Hospital, Miguel Hernandez University, No: PI2018-141. The study was included in a public registry (ClinicalTrials.gov NCT03211286) before beginning enrollment of patients.
- Tengberg PT, Foss NB, Palm H, Kallemose T, Troelsen A. Tranexamic acid reduces blood loss in patients with extracapsular fractures of the hip: results of a randomised controlled trial. Bone Joint J. 2016 Jun;98-B(6):747-53. doi: 10.1302/0301-620X.98B6.36645. Erratum in: Bone Joint J. 2016 Dec;98-B(12 ):1711-1712. PMID: 27235515.
- Nikolaou VS, Masouros P, Floros T, Chronopoulos E, Skertsou M, Babis GC. Single dose of tranexamic acid effectively reduces blood loss and transfusion rates in elderly patients undergoing surgery for hip fracture: a randomized controlled trial. Bone Joint J. 2021 Mar;103-B(3):442-448. doi: 10.1302/0301-620X.103B3.BJJ-2020-1288.R1. PMID: 33641430.
- Tian S, Shen Z, Liu Y, Zhang Y, Peng A. The effect of tranexamic acid on hidden bleeding in older intertrochanteric fracture patients treated with PFNA. Injury. 2018 Mar;49(3):680-684. doi: 10.1016/j.injury.2018.01.026. Epub 2018 Feb 2. PMID: 29426608.
- Ma H, Wang H, Long X, Xu Z, Chen X, Li M, He T, Wang W, Liu L, Liu X. Early intravenous tranexamic acid intervention reduces post-traumatic hidden blood loss in elderly patients with intertrochanteric fracture: a randomized controlled trial. J Orthop Surg Res. 2021 Feb 3;16(1):106. doi: 10.1186/s13018-020-02166-8. PMID: 33536047; PMCID: PMC7860029.
- Chen F, Jiang Z, Li M, Zhu X. Efficacy and safety of perioperative tranexamic acid in elderly patients undergoing trochanteric fracture surgery: a randomised controlled trial. Hong Kong Med J. 2019 Apr;25(2):120-126. doi: 10.12809/hkmj187570. Epub 2019 Mar 28. PMID: 30919809.
- Caron A, Depas N, Chazard E, Yelnik C, Jeanpierre E, Paris C, Beuscart JB, Ficheur G. Risk of Pulmonary Embolism More Than 6 Weeks After Surgery Among Cancer-Free Middle-aged Patients. JAMA Surg. 2019 Dec 1;154(12):1126-1132. doi: 10.1001/jamasurg.2019.3742. PMID: 31596449; PMCID: PMC6802263.
- Katsoulis M, Benetou V, Karapetyan T, Feskanich D, Grodstein F, Pettersson-Kymmer U, Eriksson S, Wilsgaard T, Jørgensen L, Ahmed LA, Schöttker B, Brenner H, Bellavia A, Wolk A, Kubinova R, Stegeman B, Bobak M, Boffetta P, Trichopoulou A. Excess mortality after hip fracture in elderly persons from Europe and the USA: the CHANCES project. J Intern Med. 2017 Mar;281(3):300-310. doi: 10.1111/joim.12586. Epub 2017 Jan 17. PMID: 28093824.
- Pedersen AB, Ehrenstein V, Szépligeti SK, Sørensen HT. Excess risk of venous thromboembolism in hip fracture patients and the prognostic impact of comorbidity. Osteoporos Int. 2017 Dec;28(12):3421-3430. doi: 10.1007/s00198-017-4213-y. Epub 2017 Sep 5. PMID: 28871320.
- Avila J, Long B, Holladay D, Gottlieb M. Thrombotic complications of COVID-19. Am J Emerg Med. 2021 Jan;39:213-218. doi: 10.1016/j.ajem.2020.09.065. Epub 2020 Oct 1. PMID: 33036855; PMCID: PMC7528743.
- Fillingham YA, Ramkumar DB, Jevsevar DS, Yates AJ, Shores P, Mullen K, Bini SA, Clarke HD, Schemitsch E, Johnson RL, Memtsoudis SG, Sayeed SA, Sah AP, Della Valle CJ. The Efficacy of Tranexamic Acid in Total Knee Arthroplasty: A Network Meta-Analysis. J Arthroplasty. 2018 Oct;33(10):3090-3098.e1. doi: 10.1016/j.arth.2018.04.043. Epub 2018 May 5. PMID: 29805106.
- Lei J, Zhang B, Cong Y, Zhuang Y, Wei X, Fu Y, Wei W, Wang P, Wen S, Huang H, Wang H, Han S, Liu S, Zhang K. Tranexamic acid reduces hidden blood loss in the treatment of intertrochanteric fractures with PFNA: a single-center randomized controlled trial. J Orthop Surg Res. 2017 Aug 15;12(1):124. doi: 10.1186/s13018-017-0625-9. PMID: 28810918; PMCID: PMC5558747.
- Mayhew D, Mendonca V, Murthy BVS. A review of ASA physical status - historical perspectives and modern developments. Anaesthesia. 2019 Mar;74(3):373-379. doi: 10.1111/anae.14569. Epub 2019 Jan 15. PMID: 30648259.
- Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994 Nov;47(11):1245-51. doi: 10.1016/0895-4356(94)90129-5. PMID: 7722560.
- Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962 Feb;51(2):224-32. PMID: 21936146.
- Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003 May;90(5):596-9. doi: 10.1093/bja/aeg111. PMID: 12697586.
- Zhou XD, Zhang Y, Jiang LF, Zhang JJ, Zhou D, Wu LD, Huang Y, Xu NW. Efficacy and Safety of Tranexamic Acid in Intertrochanteric Fractures: A Single-Blind Randomized Controlled Trial. Orthop Surg. 2019 Aug;11(4):635-642. doi: 10.1111/os.12511. Epub 2019 Aug 16. PMID: 31419080; PMCID: PMC6712408.
- Zhang S, Xiao C, Yu W, Long N, He F, Cai P, Luo K, Jiang Y. Tranexamic acid safely reduces hidden blood loss in patients undergoing intertrochanteric fracture surgery: a randomized controlled trial. Eur J Trauma Emerg Surg. 2022 Apr;48(2):731-741. doi: 10.1007/s00068-020-01387-0. Epub 2020 May 15. PMID: 32415365.
- Watts CD, Houdek MT, Sems SA, Cross WW, Pagnano MW. Tranexamic Acid Safely Reduced Blood Loss in Hemi- and Total Hip Arthroplasty for Acute Femoral Neck Fracture: A Randomized Clinical Trial. J Orthop Trauma. 2017 Jul;31(7):345-351. doi: 10.1097/BOT.0000000000000837. PMID: 28633147.
- Zufferey PJ, Miquet M, Quenet S, Martin P, Adam P, Albaladejo P, Mismetti P, Molliex S; tranexamic acid in hip-fracture surgery (THIF) study. Tranexamic acid in hip fracture surgery: a randomized controlled trial. Br J Anaesth. 2010 Jan;104(1):23-30. doi: 10.1093/bja/aep314. PMID: 19926634.
- Narkbunnam R, Chompoonutprapa A, Ruangsomboon P, Udomkiat P, Chareancholvanich K, Pornrattanamaneewong C. Blood loss and transfusion rate compared among different dosing regimens of tranexamic acid administration in patients undergoing hip hemiarthroplasty for femoral neck fracture: A randomized controlled trial. Injury. 2021 Oct;52(10):2986-2990. doi: 10.1016/j.injury.2021.08.001. Epub 2021 Aug 4. PMID: 34384597.
- Luo X, He S, Lin Z, Li Z, Huang C, Li Q. Efficacy and Safety of Tranexamic Acid for Controlling Bleeding During Surgical Treatment of Intertrochanteric Fragility Fracture with Proximal Femoral Nail Anti-rotation: A Randomized Controlled Trial. Indian J Orthop. 2019 Mar-Apr;53(2):263-269. doi: 10.4103/ortho.IJOrtho_401_17. PMID: 30967695; PMCID: PMC6415572.
- Liu W, Deng S, Liang J. Tranexamic acid usage in hip fracture surgery: a meta-analysis and meta-regression analysis of current practice. Arch Orthop Trauma Surg. 2022 Oct;142(10):2769-2789. doi: 10.1007/s00402-021-04231-1. Epub 2021 Oct 28. PMID: 34709457.
- Masouros P, Antoniou G, Nikolaou VS. Efficacy and safety of tranexamic acid in hip fracture surgery. How does dosage affect outcomes: A meta-analysis of randomized controlled trials. Injury. 2022 Feb;53(2):294-300. doi: 10.1016/j.injury.2021.09.063. Epub 2021 Oct 12. PMID: 34689986.
- Jiang W, Shang L. Tranexamic acid can reduce blood loss in patients undergoing intertrochanteric fracture surgery: A meta-analysis. Medicine (Baltimore). 2019 Mar;98(11):e14564. doi: 10.1097/MD.0000000000014564. PMID: 30882622; PMCID: PMC6426473.
- Smith GH, Tsang J, Molyneux SG, White TO. The hidden blood loss after hip fracture. Injury. 2011 Feb;42(2):133-5. doi: 10.1016/j.injury.2010.02.015. Epub 2010 Mar 16. PMID: 20236640.
- Harper KD, Navo P, Ramsey F, Jallow S, Rehman S. "Hidden" Preoperative Blood Loss With Extracapsular Versus Intracapsular Hip Fractures: What Is the Difference? Geriatr Orthop Surg Rehabil. 2017 Dec;8(4):202-207. doi: 10.1177/2151458517729615. Epub 2017 Nov 22. PMID: 29318081; PMCID: PMC5755838.
- Xiao C, Zhang S, Long N, Yu W, Jiang Y. Is intravenous tranexamic acid effective and safe during hip fracture surgery? An updated meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg. 2019 Jul;139(7):893-902. doi: 10.1007/s00402-019-03118-6. Epub 2019 Jan 14. PMID: 30637503.
- Myles PS, Smith JA, Kasza J, Silbert B, Jayarajah M, Painter T, Cooper DJ, Marasco S, McNeil J, Bussières JS, McGuinness S, Byrne K, Chan MTV, Landoni G, Wallace S, Forbes A; ATACAS investigators and the ANZCA Clinical Trials Network. Tranexamic acid in coronary artery surgery: One-year results of the Aspirin and Tranexamic Acid for Coronary Artery Surgery (ATACAS) trial. J Thorac Cardiovasc Surg. 2019 Feb;157(2):644-652.e9. doi: 10.1016/j.jtcvs.2018.09.113. Epub 2018 Oct 19. PMID: 30459103.
- Shi J, Wang G, Lv H, Yuan S, Wang Y, Ji H, Li L. Tranexamic Acid in on-pump coronary artery bypass grafting without clopidogrel and aspirin cessation: randomized trial and 1-year follow-up. Ann Thorac Surg. 2013 Mar;95(3):795-802. doi: 10.1016/j.athoracsur.2012.07.015. Epub 2012 Sep 7. PMID: 22959576.
- Gao FQ, Li ZJ, Zhang K, Sun W, Zhang H. Four Methods for Calculating Blood-loss after Total Knee Arthroplasty. Chin Med J (Engl). 2015 Nov 5;128(21):2856-60. doi: 10.4103/0366-6999.168041. PMID: 26521781; PMCID: PMC4756876.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
