Annals of Musculoskeletal Medicine
Fibromyalgia (FM): Definition, Differential Diagnosis, Clinical Contexts and Therapeutic Approaches. The Recent State of the Medical Art and a Possible Key to Etiological Interpretation. A Narrative Review
Giulio Perrotta*
Department of Psychology, Universitas Mercatorum, Rome, Italy
Cite this as
Perrotta G. Fibromyalgia (FM): Definition, Differential Diagnosis, Clinical Contexts and Therapeutic Approaches. The Recent State of the Medical Art and a Possible Key to Etiological Interpretation. A Narrative Review. Ann Musculoskelet Med. 2025: 9(1): 001-011. DOI: 10.17352/amm.000035Copyright Licence
© 2025 Perrotta G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Fibromyalgia (FM) is a syndrome currently considered idiopathic and multifactorial rheumatic syndrome that causes increased muscle tension, especially during the use of the same muscles. It is characterized by muscle and fibrous tissue (tendons and ligaments) pain of a chronic type associated with stiffness, asthenia (decline in strength with fatigability), cognitive impairment, insomnia or sleep disturbances, and altered sensitivity to stimuli. The etiology is currently unknown. It is under discussion whether to retain it in the definition of the atypical rheumatic syndrome. The hypotheses are oriented according to a multifactorial logic, immunoreumatological in nature with neurological, psychiatric, muscular and metabolic symptoms, although (being a diagnosis by exclusion) it tends to include known phenomena (such as psychiatric symptoms) in a spectrum of heterogeneous symptoms, thus risking diagnostic error, even to accommodate the patient’s needs and wants. This review provides the reader with a comprehensive overview of the disorder, highlighting some aspects that could redefine its clinical framing, thus facilitating a better diagnostic approach to the health problem, from an etiological perspective.
Abbreviations
FM: Fibromyalgia; FMS: Fibromyalgia Syndrome; HLA: Human Leukocyte Antigen; CFS: Chronic Fatigue Syndrome; MCS: Multiple Chemical Sensitivity; SFB: Benign Fasciculations Syndrome or Benign Fasciculations Syndrome
Introduction
Definition, etymology and epidemiology
Fibromyalgia (FM), also called Fibromyalgia Syndrome (FMS) or Atlas syndrome, is a syndrome currently considered a multifactorial idiopathic disorder of inflammatory nature on a genetic basis, with clinical manifestations of immunoreumatic, neurological, psychiatric, muscular, and metabolic [1-4]. The term fibromyalgia comes from the Latin “fiber” and the Greek “myo” (muscle), combined with algos (pain) [5]. In the past, as far back as the 1800s, the disease was already known, but by many other names: in 1904 for example, the disease was called Fibrosite by William Richard Gowers. Federigo Sicuteri identified in the 1960s the figure of the pain syndrome now known as Fibromyalgia. He named it “Panalgesia” (pan=all, algesia=pain) and submitted this nosological figure to the IASP College, which recognized the dignity of disease to this condition, but renamed it with the Anglophone name of “Fibromyalgia”. Federigo Sicuteri had already shed light on its origin with animal experiments. The mechanism of origin was defined as serotonergic and NMDA-related [6,7].
It mainly arises in females in adulthood (second to fifth decades, peaking around 25-35 and 45-55 years of age), although there are rather rare cases of fibromyalgia in pediatric age or during adolescence, probably due to diagnostic error or symptomatological overlap. Its prevalence in the rheumatologic disease group turns out to be between 12% and 20%; in the general population, it is 0.5% in males and 3.5% in females. Other studies limit it to 2-8% of the population, with female-to-male incidence between 7:1 and 9:1. Diagnosis often comes late and after many medical checks, as it is often misinterpreted. There exists in the literature, however, several cases of fibromyalgia in pediatric and adolescent patients, although the incidence appears extremely low compared to the adult population. The pain, fatigability, and functional difficulties due to the symptoms can lead the person with the condition to isolation from work, group, and emotional life, in part because he or she is often misjudged as “hypochondriacal” or exaggerated in focusing on symptoms; however, it is estimated that fibromyalgia may be misdiagnosed in 75% of people [8-11].
Aims
This review provides the reader with a comprehensive overview of the disorder, highlighting some aspects that could redefine its clinical framing, thus facilitating a better diagnostic approach to the health problem, from an etiological perspective.
Materials and methods
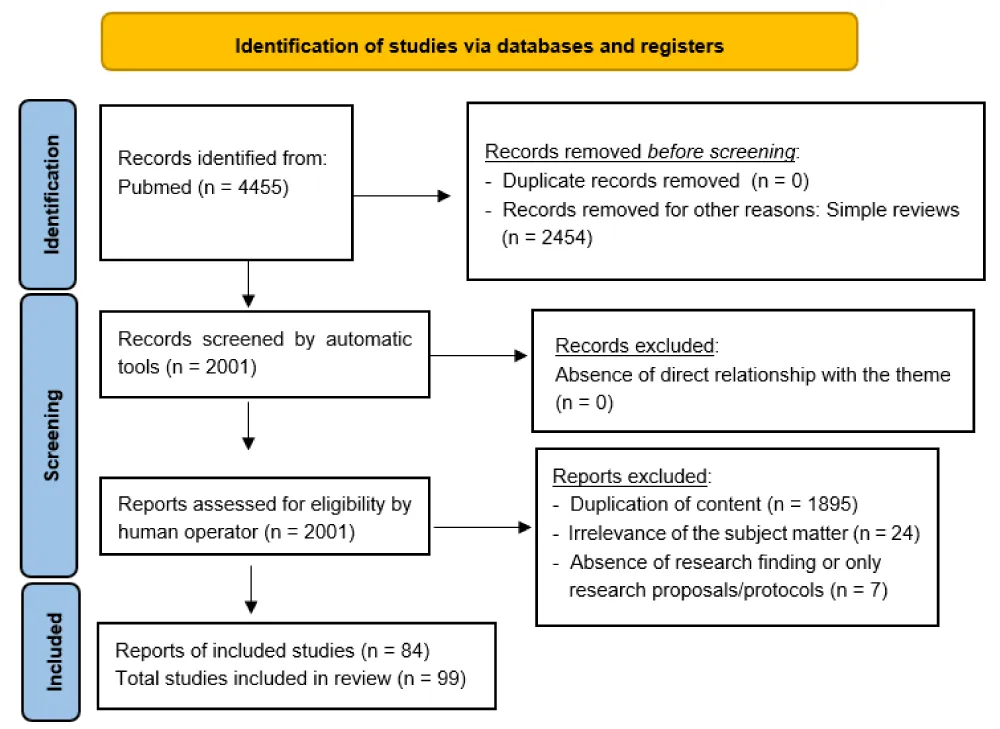
The author searched PubMed, from January 1964 to May 2025, for meta-analyses, clinical trials and randomized controlled trials, using “Fibromyalgia”, for this narrative review, selecting 2001 eligibility results of which 84 were included by removing duplicate content, irrelevant items, and absence of search items. Then, to get an even broader and more complete overview of the topic, 15 more references were added, for a total of 99 results. Simple and systematic reviews, opinion contributions, or publications in popular volumes were excluded because they were not relevant or redundant for this work. The search was not limited to English-language papers (Figure 1).
Clinical profiles
Diagnostic criteria and main symptoms of clinical relevance
The diagnosis of FM is perfected by exclusion, based on the patient’s self-reported symptoms in the history and during the clinical interview, supplemented by the results of instrumental tests. Symptoms generally stated are fatigue, algic hypersensitivity, and fasciculation, which are common to 3 other syndromes often accumulated with FM and not infrequently considered a single clinical entity: Chronic Fatigue Syndrome (CFS), Multiple Chemical Sensitivity (MCS), and Benign Fasciculations Syndrome or Benign Fasciculations Syndrome (SFB). The organs most affected by this syndrome are the musculoskeletal system (algesia, contractures, hypertone/hypotone, inflammation, stiffness, muscle tension, asthenia, twitch, cramps, joint and spine pain, and paresthesia), the nervous and sensory systems (sleep rhythm disturbances, dystonias, tingling, photosensitivity, tinnitus, temporary states of confusion or lightheadedness, concentration deficits, headache, balance alterations, altered temperature perception, altered mood and anxiety states, meteoropathy, restless legs syndrome and dysregulation of the hypothalamus-pituitary-adrenal -HPA- axis), the gastrointestinal and urogenital systems (reflux, nausea, colitis, irritable colon, dry mouth, cystitis, vestibulitis, dysmenorrhea, and hormonal alterations), the lymphatic and hematopoietic systems (clotting defects, Raynaud’s phenomenon, and intolerance to temperature changes) and the tegumentary system (itching, redness, and dermo-allergic symptoms, also brought about by intolerances and allergies), with the algic manifestation at all levels and for effects identical to neuropathic pain of an inflammatory nature [12,13].
Thus, having excluded neurological, musculoskeletal and immunoreumatic, vascular, and hormonal pathologies, the therapist must clinically approach the symptom picture to aid the diagnosis, which will be fibromyalgia by exclusion; in these patients, the most persistent complaint is that referring to perceived pain, which in them may present itself in various ways (from a constant slight soreness to aching or sharp pain in certain areas) but is nevertheless localized and accentuated at focal points called “tender points” (i.e., points where localized pain can be evoked by acupressure), not to be confused with “trigger points” in which pain is not well localized but tends to be perceived as radiating to a neighboring area [14].
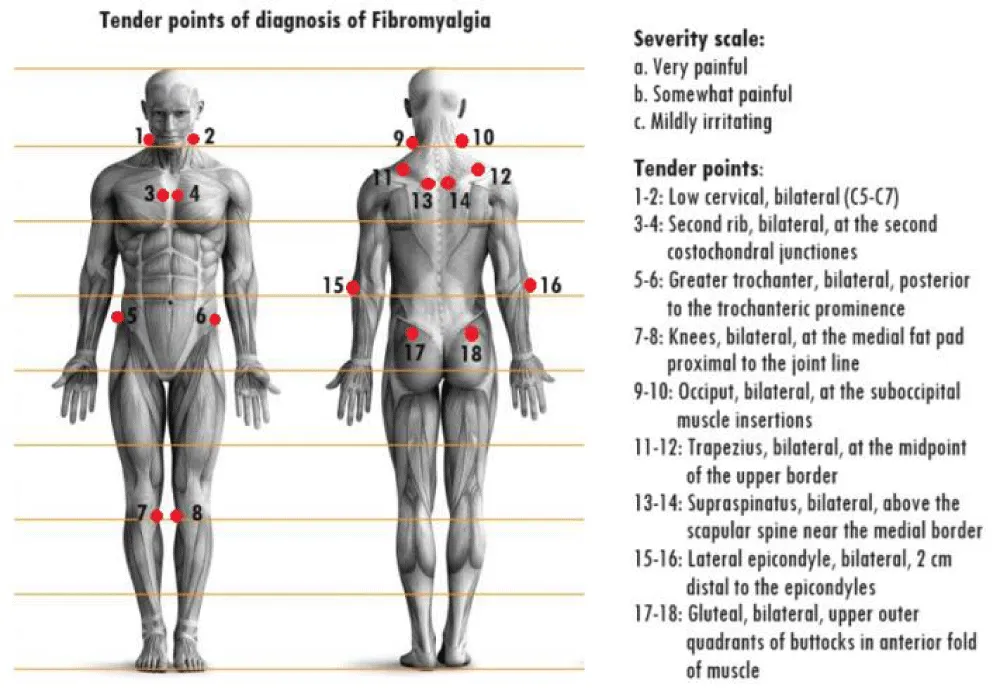
A careful history from which the exclusion of other pathologies as descriptive causes of the symptomatological picture is inferred (a), pain is inferred to have persisted for at least 3 months (b), and the patient declares palpation of at least 11 to 18 key points (tender points) applying pressure of at least 4 kg (c) as painful, may be suggestive for the diagnosis of FM. If they turn out to be fewer than 11 but meet the other criteria for fibromyalgia (widespread pain, muscle stiffness, unrestful sleep), it would be good to still pursue treatment for fibromyalgia. The key points (Figure 2) are located (each point treats both the right and left sides): 4 in the anterior neck, 4 behind the shoulders, 2 at the height of the cerebellum (suboccipital intersection of the trapezius), 2 at the height of the elbows, 2 at the height of the knees, 2 above the buttocks, and the last 2 at the lower sides of the buttocks (retro trochanteric region) [15](Table 1).
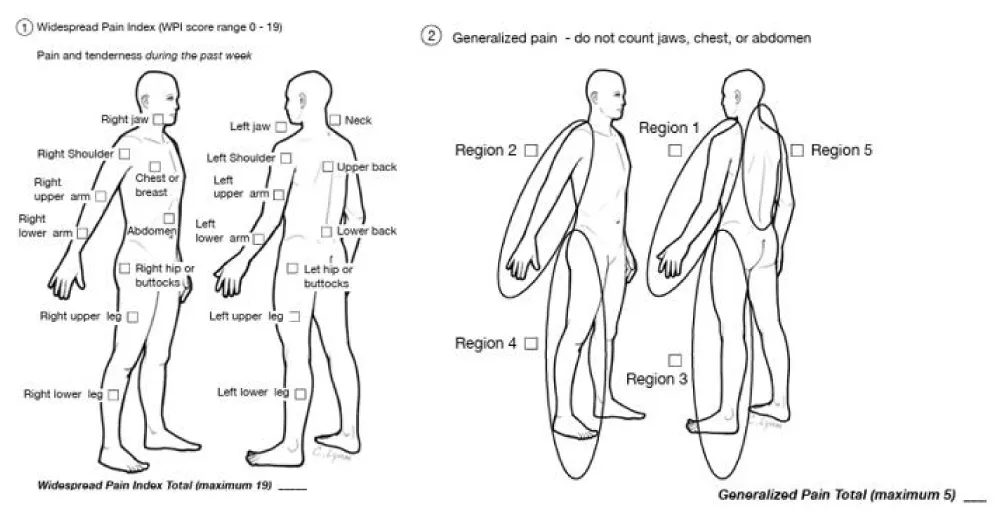
Several diagnostic criteria for FM have been proposed in recent years, including the revised 2016 American College of Rheumatology (ACR) criteria, the criteria of the ACTTION-American Pain Society Pain Taxonomy (AAPT) group, and the modified 2019 Fibromyalgia Assessment Status (FAS) criteria. Despite the appearance of newer criteria for FM diagnosis, the 2016 ACR criteria demonstrate the best performance [16,17] (Table 2) (Figure 3).
Etiology
The exact cause of FM is still unknown, and the most accepted hypothesis seems to be multifactorial, in the absence of specific confirmation. However, the breadth of symptoms suffered would suggest a disorder of a genetic-based inflammatory nature [18-21], capable of affecting multiple body districts; in fact, research has found that:
Concerning psychiatric symptomatology, high vulnerability to stress facilitates dysfunctional humoral and anxiety-like overactivations, also interfering with the perceptual state and promoting circadian rhythm sleep disturbances [22-24]. Other abnormalities found are related to the hippocampus and low concentration of N-acetyl aspartate (NAA), confirming a neurometabolic relationship [25].
Concerning neurological symptomatology, one study demonstrated excessive innervation in the hands, affecting the nerves involved in regulation (opening and closing), caused by local arteriovenous shunts (relevant arteriovenous malformation). In particular, they remain excessively open, preventing proper perfusion to the tissues. As a result, there is hypoxia, and thus pain, reduced muscle strength, and impaired thermoregulation. Cold is one of the most damaging and painful components for the patient. Sympathetic hyperactivity results particularly in alterations in peripheral and central microcirculation, such as altered distribution of capillaries at the level of muscle tissue, with hypervascularization of tender points, presence of Raynaud’s phenomenon, alterations in cerebral flow with decreased flow in particular brain areas (caudate nucleus and thalamus) responsible for the transmission and modulation of pain. A nuclear medicine study would correlate some clinical manifestations of the syndrome with perfusion alterations in the encephalic areas deputed to the perception and emotional processing of nociceptive stimuli, whereby perception is impaired [26]. Alterations in several neurotransmitters are demonstrated and confirmed, proving the origin in the central nervous system of fibromyalgia, especially to serotonin, norepinephrine, and dopamine, which could cause or aggravate the hyperactivity of the neurovegetative nervous system (which controls by reflex mechanisms numerous functions of the body including muscle contraction, but also sweating, vasodilation, and vasoconstriction) [27]. According to other studies, patients with fibromyalgia have lower or similar cognitive functions (such as long-term memory and work capacity) compared with subjects older than 20 years; they may present similar performance to healthy subjects only with extensive activation of the frontal and parietal regions, i.e., with greater expenditure of energy. It appears that there is 3.3 times more gray matter loss than in healthy peer subjects [28-30]. Recently then a central role for dopaminergic neurotransmission in pain perception has been demonstrated, so in patients with fibromyalgia or chronic fatigue syndrome, a decrease in dopamine in the CNS (as occurs in the substantia nigra in cases of Parkinson’s disease) probably contributes to the onset of the painful symptoms that occur in fibromyalgia, as well as to small involuntary muscle movements, mild hypertone, restless legs syndrome, and stiffness [31].
Concerning immunoreumatic and musculoskeletal (and therefore inflammatory) symptomatology, a key role of neurogenic inflammation has been hypothesized for some years. This is a difficult-to-diagnose inflammation derived from the localized release by afferent neurons of inflammatory mediators such as substance P, CGRP, neurokinin A (NKA), nitric oxide, vasoactive intestinal polypeptide, to a lesser extent Serotonin 5-HT (other serotonin neurotransmitters are deficient) and endothelin-3 (ET-3). TRPA1 channels stimulated by lipopolysaccharide (LPS) can also cause acute neurogenic inflammation. Once released, neuropeptides induce histamine release from adjacent mast cells. In turn, histamine evokes the release of substance P and CGRP; a bidirectional link between histamine and neuropeptides in neurogenic inflammation is subsequently established. Histamine also causes allergic reactions and the increase, visible on examination, of eosinophilic white blood cells that degrade it. Neurogenic inflammation, identified in recent studies, appears to play an important role in the pathogenesis of numerous diseases, including migraine, psoriasis, vitiligo, asthma, fibromyalgia, eczema, vasomotor rhinitis, rosacea, allergy, dermatitis, dystonia, and multiple chemical sensitivity. It may result from an initial magnesium deficiency or nerve trauma: many fibromyalgia patients have pre-existing chronic disorders of the temporomandibular joint, for example, and in migraine, local stimulation of the trigeminal nerve (which also includes the mandibular and maxillary nerves) during testing has been found to cause systemic neurogenic inflammation through the release of neuropeptides. Cervical arthrosis and osteoarthritis are risk factors [32-35].
Other etiologic hypotheses are being discussed in the literature but there is insufficient data. All of the following causes, however, can be considered as activating factors for fibromyalgic pathology (if we consider it to be a genetically based condition) but certainly not to be etiologic hypotheses per se as erroneously considered, since the pathologic force of such circumstances is acute and not chronic; they pertain to the onset of physical trauma of a musculoskeletal nature (injuries or conditions such as arthrosis and radiculopathy), psychic trauma, a febrile illness of viral etiology (particularly from Epstein-Barr virus, or influenza virus) or bacterial of celiac disease or other allergies including the form of gluten sensitivity, of sharing specific metabolic phenotypes, or of other inherited neuropathies such as tomacular or axonal or multifocal motor neuropathy even mild or Guillain-Barré, or even of mitochondrial channelopathies and alterations such as congenital or immunomedially induced dysfutions of proteins deputed to ion exchange (ion channels), thus a congenital myopathy (myotonic channelopathies) or acquired (by autoimmunity as in Lambert-Eaton syndrome or Isaacs syndrome) of difficult diagnosis, with involvement of sodium channels (especially the one called Nav1. 7), or calcium or potassium, causing pain and nerve or muscle fiber problems with easy fatigability. In a percentage of patients, the presence of alterations in monocyte mitochondria present in the blood, muscle cells, or skin tissues, with the reduction in mass due to cellular autophagy caused by the lysosome, with no apparent presence of a genetic mitochondrial myopathy among those currently identified or metabolic involvement, has been revealed by extensive histologic analysis [31,36-42].
On the other hand, the role of peripheral small-fiber neuropathy (also suspected of benign fasciculations syndrome), a difficult-to-diagnose polyneuropathy that in 82% of cases has a normal EMG and nerve conduction analysis result (and sometimes only visible by biopsy) [43], and the role of the gut microbiota (whether therefore it is dysbiosis that triggers or facilitates fibromyalgia or whether fibromyalgia determines dysbiosis) [44-47].
The controversial and ill-defined role of cytokines in patients diagnosed with FM is yet to be clarified and may be related to inflammatory phenomena arising from other diseases or atypicality of already known but incorrectly diagnosed diseases [48] (Table 3).
Candidate gene studies of fibromyalgia
In the literature, studies dedicated to analyzing correlations between FM and genetics have used low-resolution DNA-based typing (polymerase chain reaction/specific oligonucleotide probe) in patients and family members for and in family members, but the small population samples could cast doubt on the validity of the results, and thus their clinical use. Having ascertained this inherent limitation, studies have shown that there is a correlation between polymorphisms of HLA A*29 and B*44 haplotypes, correlating more closely with patients diagnosed with FM to those with Sjögren’s syndrome, Interstitial cystitis, Hypothyroidism, Systemic lupus erythematosus, Mixed connectivitis, Chronic fatigue syndrome, Rheumatoid arthritis, and Raynaud’s phenomenon. The DRB1 and DR4 haplotypes, the 102T/C polymorphism of the 5-HT2A receptor, and the ADRB2, ADRA1A, COMT rs4818, and MAO-A allele3 polymorphisms also turn out to be affected; finally, increased peripheral levels of Brain-Derived Neurotrophic Factor (BDNF) have also been correlated with FM [16-21].
Types and comorbidities
There is no agreement in the literature on the different types of fibromyalgia, as there are many differences in the nervous system profiles and among individuals on the psychophysical level and thus may indicate the existence of different subtypes of fibromyalgia. A 2007 study [49] not confirmed by other studies but not disputed rationalized the issue by identifying 4 different types:
“FORM A”: extreme sensitivity to painful stimuli but without associated psychiatric conditions (may respond to drugs that block serotonin or 5-HT3 receptors, such as SSRIs, or antiepileptic or antiparkinsonian drugs);
“FORM B”: fibromyalgia with comorbid psychiatric symptoms, depression with pain (may respond to SSRI antidepressants);
“FORM C”: major depression with concomitant fibromyalgia syndrome (very responsive to SSRIs);
“FORM D”: fibromyalgia due to somatization (patient may also benefit from psychotherapy in addition to SSRIs and other psychotropic drugs).
In the writer’s opinion, however, this classification appears to be reductive and not very adherent to the clinical reality known today, and this aspect will be the subject of an upcoming research paper.
It is common that the symptomatology described by a patient with a presumed diagnosis of fibromyalgia recalls other morbidities, and that some of these coexist, in a comorbid relationship, as is the case with chronic fatigue syndrome (about 70% of fibromyalgia patients also manifest CFS criteria) and restless legs syndrome, or even some autoimmune syndromes such as allergies, arthritis and psoriasis, and some neuropathies, including carpal tunnel syndrome, but also herniated discs, discopathy, radiculopathy, and tendinopathy, precisely by the markedly inflammatory nature of FM, while under the psychiatric profile correlation is found with anxiety and mood states, somatization, and various sleep disorders, precisely by the inability to manage the pain and symptomatology described, causing a sense of helplessness and resignation [50].
Instrumental analysis and differential diagnosis
There are no unambiguous biological markers for the diagnosis of FM, but there are indicators that can help the diagnosis, such as is the case with the evaluation of CBC and leukocyte distribution outcomes (e.g., a marked presence of eosinophils or monocytes, as it comes in inflammatory and infectious processes) [51,52], 5-hydroxy-tryptophan concentration in CSF and plasma (e.g., a reduced presence, as occurs in humoral and depressive disease states and pain regulation) and melatonin (e.g., a reduced decrease, as occurs in sleep disorders); other blood tests, such as Creatine Phosphokinase (CPK) or instrumental tests such as electromyography, on the other hand, have given conflicting results, and are usually therefore only used to rule out myopathies, neuronal damage, and neuropathies in differential diagnosis. Therefore, tests for erythrocyte sedimentation rate, C-reactive protein, antinuclear antibodies, CBC with leukocyte formula, creatine phosphokinase, transaminases, anti-Epstein-Barr virus, anti-gliadin and anti-hepatitis C antibodies, thyroid-stimulating hormone, thyroxine, rheumatoid factor, anti-parietal cell antibodies and anti-intrinsic factor antibodies are usually performed to rule out many diseases with specific markers or indicators. Objective examination remains the most valid examination [53].
Based on the patient’s medical history and the outcome of instrumental examinations prescribed by the therapist, the diagnosis must be refined by excluding pre-existing, concomitant, or absorbing physical and/or mental illnesses: obesity, immunoreumatic diseases, such as Sjögren’s syndrome, rheumatoid arthritis, and systemic Lupus erythematosus; rheumaticovascular diseases, such as vasculitis; neurodegenerative and neurovascular diseases, such as dementia, multiple sclerosis, neuropathies, and algic syndromes; musculoskeletal diseases, such as myopathies, cartilage and joint dysfunction; gastrointestinal, metabolic, and hormonal diseases, such as thyroiditis, recto-ulcerative colitis, and chronic gastritis; allergic diseases, such as celiac disease, gluten intolerance, and specific allergies; oncological disease; bacterial and viral infections [54].
Prognosis
Although FM is not a degenerative or fatal condition, the pain perceived by the patient is pervasive and disabling, with symptoms often described as fluctuating to numerous external factors that are capable of causing it to worsen: there is evidence of an influence of climatic factors (pain and stiffness worsen in spring, autumn, and periods of high humidity), hormonal factors (premenstrual period, thyroid dysfunction), psychological stressors, and aging, but also by working conditions, economic problems, and family stress. For these reasons, symptomatology is highly variable in both quantity and quality and can have periods of remission, especially if appropriate treatment is followed [55].
Therapeutic approaches and prognosis
Treatments for fibromyalgia are varied, and the correct one is the approach that takes into account the patient’s medical history and the tests performed to rule out other conditions. At that point, specific therapy is proceeded according to the persistence, intensity, and complexity of the symptom [56,57].
Pharmacological therapies
Given the impossibility of making an evidence-based diagnosis, and especially because of the equivocal nature of fibromyalgia, there is no universally adopted therapy whose efficacy is scientifically proven [3]. Many drugs are used, such as nonsteroidal anti-inflammatory drugs (NSAIDs), but they only serve to contain inflammation with even serious side effects; many more encouraging results have come from the mindful use of muscle relaxants (such as cyclobenzaprine) and S-adenosyl-methionine, which unlike central muscle relaxants (oxybutynin, succinylcholine, vecuronium, silodosin, or anxiolytics such as benzodiazepines) have temporary efficacy, in that after mild alleviation of painful symptoms, they accentuate the cognitive and perceptual deficits often present in the syndrome. Other studies have demonstrated the efficacy of antidepressants such as Amitriptyline (a tricyclic antidepressant), which remains the active ingredient of first choice, and of Fluoxetine (selective serotonin reuptake inhibitor more commonly called SSRIs) and Duloxetine, with fair results always in the short term. In addition, venlafaxine, which is an antidepressant that acts on both serotonin and norepinephrine, is also used, as well as sertraline and paroxetine [58]. Melatonin, a sleep-related hormone, can improve insomnia symptoms as a result of pain [59]. Antiepileptic drugs (such as clonazepam, gabapentin, or pregabalin), central opioid analgesics (tramadol and codeine/paracetamol), and some antiparkinsonian drugs (often used against pain and spasticity) such as pramipexole may also be administered in certain cases: pregabalin, in particular, is an analogue of the neurotransmitter GABA, like gabapentin, and is indicated in the treatment of peripheral neuropathic pain, i.e., due to an anatomical and/or functional abnormality of the peripheral nervous system’s pain-signaling mechanism, but gives appreciable results only on a minority percentage of fibromyalgia patients; gabapentin, pregabalin, and pramipexole are reserved for severe cases and short periods, as heavy side effects such as drowsiness, cognitive impairment, altered heart rate, vomiting, tremors, edema, psychic problems, hypotension, and paradoxical hyperalgesia (pain exacerbation) are very common [60]. Opioids, apart from codeine and tramadol, play a marginal role in the treatment of fibromyalgia, as they act on pain pathways at the level of the central nervous system that is not affected in this syndrome, which uses other pathways of pain transmission and control, as yet totally unknown. They also have side and undesirable effects and induce tolerance and dependence. Use, however, is spottily approved: if use is approved in the United States of America, in Europe only tramadol and lidocaine are recommended, while strong opioids (e.g., hydrocodone, oxycodone, and morphine) are not recommended for their collaterals even deadly effects, especially when taken in combination with other drugs or with each other [61,62]. Some sufferers have benefited then from very low-dose dopaminergic (used for Parkinson’s motor symptoms), such as the aforementioned pramipexole or levodopa. It would be inadvisable (although in some cases they are used at a low dose for symptoms such as insomnia and pain) therefore, according to the dopaminergic hypothesis the long-term use of neuroleptics (dopamine antagonists, which can cause extrapyramidal effects, and increase some symptoms of the disease), especially in medium to high dosage, in fibromyalgia patients who have comorbidities of mental disorders [63]. A recent study showed that duloxetine is associated with greater efficacy for treating pain and depression, while amitriptyline is associated with greater efficacy for improving sleep, fatigue, and overall quality of life [64]. The therapeutic strategy is being advanced with the use of cannabinoids for medical purposes, due to their analgesic, muscle-relaxing, antidepressant, and anxiolytic effects and improved sleep quality; also of note are the fewer side effects compared to other molecules, the low physical dependence (although psychological dependence cannot be ruled out), and the reduced tolerance developed even after prolonged periods of use compared to antidepressants [65-67]. Finally, corticosteroids, once used very easily for their anti-inflammatory properties, are now contraindicated except in low doses because of their impact on the immune system, as are immunosuppressants since the autoimmune genesis of FM disorder is not clear in the literature and guidelines [68].
Nonpharmacological therapies or supportive
The patient diagnosed with FM can improve their health status by also acting on their lifestyle. The patient should also avoid doing work that is too physically demanding, as well as residing in warm and dry environments. Physical activity, sleep recovery, relaxation exercises and physiotherapy, specific coaching paths and a healthy, balanced diet are the basis of this nonpharmacological intervention. Specifically: constant, not overly intensive and progressive physical activity increases muscle elasticity, detoxifies the body and hardens the immune system, promoting systemic disinflation; relaxation exercises and personalized coaching pathways promote a positive impact on certain neurotransmitters, such as serotonin, and specific techniques such as autogenic training, hypnotherapy, mindfulness and yoga, have been shown to be effective in decreasing the subjective algic perceptual state; healthy, balanced diet, managed by a licensed nutritionist, promotes the process of toxin release by decreasing the overall inflammatory state (in particular, eliminating animal protein, refined carbohydrates and acidifying foods, also limiting tomatoes, potatoes, eggplant and peppers, or even favoring a vegetarian diet and in any case before gluten and lactose with supplemental intake especially of vitamin B-complex, C, E, D3, magnesium, omega 3-6, coenzyme Q10, palmitoylethanolamide and acetyl-L-carnitine) [46,47,69-78].
Other treatment options have also been investigated in the literature that have shown good efficacy without clinically significant side effects, especially on algic symptoms, as in the case of ozone therapy, ultrasound, transcranial direct current stimulation (tDCS) albeit with lukewarm evidence, whole-body cryotherapy (WBC) and low-level laser therapy (LLLT) [79-82].
Also in the literature, there is ample room for studies on psychological treatments, especially cognitive-behavioural therapy and mindfulness approach techniques, which are effective in both quantitative diminutions of algic symptoms and improvement of the subjective perceptual state in qualitative terms, including mood fluctuations and anxious and neurotic states (as occurs in phobic and obsessive manifestations, also related to one’s bodily perceptual state) that pertain to one’s personality profile even before the presumed diagnosis of fibromyalgia. That fibromyalgia patients have a strong psychological relapse is also shown by placebo effect studies [83-90].
Alternative medicine therapies
In the literature, we also find a space for discussion of the use of alternative medicine therapies, thus nontraditional/western, and although legitimate doubts have been raised about the scientific methodological rigor used, the results appear to be promising in terms of efficacy, whether using homeopathy [91], acupuncture (and dry needling) [92-98], or even traditional Chinese medicine [99], all of which aim to impact the high inflammatory levels that generate the algic symptoms. Generally, fibromyalgia is considered a widespread musculoskeletal pain disorder, with greater prevalence in the female population, and with symptoms of asthenia, somnolence, headache, osteoarticular disorders, gastrointestinal symptoms and cognitive-behavioural alterations, which begin after psychophysical trauma. But if in biomedicine, the basic therapy is on pharmacological administration of antidepressants, painkillers, antiepileptics and sleep inducers to lessen the symptomatic impact on the patient’s subjective perception, along with dietary diet, supplements, sports activity and body therapies, in alternative medicine therapies the approach is markedly different:
In homeopathy, remedies used are Arnica montana for aches and bruises, fatigue and numbness, Rhus toxicodendron for osteo-articular, musculoskeletal pain and body stiffness, but also to detoxify the body, Bryonia Alba for pain resulting from movement, Magnesium Phosphoricum for acute muscle and joint pain, Ruta Graveolens for restlessness, Arsenicum album for fatigue, Cimicifuga Racemosa for stiffness and muscle pain, Actea Racemosa for muscle and nerve pain, Gelsemium Sempervirens for fatigue and muscle pain, Coffea Cruda for sleep problems, Ignatia Amara for depression and bereavement, Causticum for widespread muscle and osteo-articular pains, Phosphoricum Acidum for feelings of fogginess and bewilderment, and Vibronica for fatigue and pain.
In traditional Chinese medicine and acupuncture, several possible protocols are identified, two of which are offered below in table (Table 4).
Controversial profiles
The definition of FM is relatively recent and continues to be called a controversial diagnosis or at any rate, an umbrella term that encompasses a heterogeneous multitude of symptoms, varying over time based on constantly changing factors, and thus leaving little room for intervention in rationalizing the subject. The same author who first set diagnostic guidelines for FM in 1990 had to retract in subsequent years, stating in 2008 and 2013 that it should no longer be considered a disease but mostly a psychophysical response to depression and stress (thus a reaction to negatively impacting circumstances on health); this stance stemmed from the realization that the etiology is rather “controversial” (even today) and “there are numerous factors that produce such symptoms and there is no consistent continuum among them”.
In literature, then, there is also a tendency not to consider fibromyalgia as a disease with its dignity, due to the lack of instrumental findings and the absence of an objective diagnostic test [55] and a logical diagnostic pathway (being a diagnosis by exclusion), although, nevertheless, recent studies indicate a correlation with some polymorphisms that have not yet been fully clarified [16-21].
It seems evident that the difficult nosographic placement (a), the often numerically insufficient population selection to arrive at the representativeness necessary to be able to consider the results of the studies reasonably functional to the research objectives (b), the heterogeneity of symptoms among them not coherently connected according to a logical continuum (c), the absence of a precise and shared independent diagnostic procedure and not according to the logic of exclusion (d), the absence of one or more specific marker (e.g. prostate-specific antigen in prostate cancer) or an instrumental test (e.g., blood eosinophil count in allergic reactions or the electromyography in muscle diseases) capable of making the diagnosis with certainty (e), the still controversial and ill-defined role of cytokines in patients diagnosed with FM (f), the significant impact of stressors on the perceptual status of algic symptoms (g) and the lability of the symptoms, which tend to change over time without a definite gravitating or progressive pattern (h), are all elements that might argue for a downsizing of the evaluative framework of this disorder.
Diagnosis by exclusion, on the other hand, complicates the interpretive and diagnostic process, making it dependent on the therapist’s evaluations without a solid and certain logical-scientific basis, other than the reductionistic one; moreover, fibromyalgia symptomatology has so many points in common with dozens of other morbid conditions that the boundary is so blurred that one can have the legitimate doubt that the clinical evaluation may be marred by a misrepresentation of all known elements, perhaps only because some are not known (e.g. symptoms omitted or misinterpreted by the patient) or knowable (e.g., atypical form of an already known disorder or consequence of a polymorphism still unknown or not properly investigated). Due in part to these complexities, it is necessary for each nation to have a specific educational plan on the topic of fibromyalgia in its health care program in order to facilitate the correct methodological and informational approach for patients, also with a view to saving money for the national health care system.
Conclusion
Fibromyalgia (FM) has an etiology that is still not investigated and that the breadth of symptoms described, and the critical issues raised in this paper leave ample room for work on the topic to frame this disorder more structurally and functionally. In the opinion of the writer, taking into account that the symptomatology is markedly somatic and not otherwise explicable except in an atypical or otherwise framed condition, it seems more likely that fibromyalgia can and should be considered as the active manifestation of a systemic inflammatory state capable of interfering with normal organic functioning and alternating one or more biological functions, resulting in the symptomatological consequence often described by patients, but which does not fit into a precise nosographic framework. The symptomatological manifestation of the psychological and psychiatric matrix then suggests that the somatic component in the patient plays a central role, both in an interpretative and therapeutic key. The future perspective must therefore be oriented on the analysis of a structured protocol functional to the objectives of diagnosis, using a representative sample and working on the exact nosographic placement of the disorder under investigation.
- Ambrose KR, Gracely RH, Glass JM. Fibromyalgia dyscognition: concepts and issues. Reumatismo. 2012;64(4):206-15. Available from: https://doi.org/10.4081/reumatismo.2012.206
- Malattia C, Chiarella L, Sansone M, Pistorio A, Lavarello C, Carpaneto M, et al. Sleep and Sleep Complaints in Juvenile Fibromyalgia Syndrome. J Rheumatol. 2023;50(6):827-834. Available from: https://doi.org/10.3899/jrheum.220720
- NIAMS.NIH.GOV. Fibromyalgia. [cited 2023 Dec 26]. Available from: niams.nih.gov/health-topics/fibromyalgia
- Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311(15):1547-1555. Available from: https://doi.org/10.1001/jama.2014.3266
- Treccani. Fibromialgia. [cited 2023 Dec 26]. Available from: treccani.it/enciclopedia/fibromialgia/
- Veniani ER, Poli EF. Fibromialgia. Anima Ed.; 2020.
- Bottaccioli F, Bottaccioli AG. Psiconeuroendocrinoimmunologia e scienza della cura integrata. Il manuale. Edra Ed.; 2017. Available from: https://www.lospallanzani.it/wp-content/uploads/2018/06/Bottaccioli_31maggio2018.pdf
- Todesco S, Gambari PF, Punzi L. Rheumatic diseases. McGraw-Hill Ed.; 2007.
- Chakrabarty S, Zoorob R. Fibromyalgia. Am Fam Physician. 2007;76(2):247-254. Available from: https://pubmed.ncbi.nlm.nih.gov/17695569/
- Bartels EM, Dreyer L, Jacobsen S, Jespersen A, Bliddal H, Danneskiold-Samsøe B. Fibromyalgia, diagnosis and prevalence. Are gender differences explainable? Ugeskr Laeger. 2009;171(49):3588-92. Available from: https://pubmed.ncbi.nlm.nih.gov/19954696/
- Clauw DJ, Arnold LM, McCarberg BH. The science of fibromyalgia. Mayo Clin Proc. 2011;86(9):907–11. Available from: https://doi.org/10.4065/mcp.2011.0206
- Beiner E, Lucas V, Reichert J, Buhai DV, Jesinghaus M, Vock S, et al. Stress biomarkers in individuals with fibromyalgia syndrome: a systematic review with meta-analysis. Pain. 2023;164(7):1416-1427. Available from: https://doi.org/10.1097/j.pain.0000000000002857
- Boomershine CS. Fibromyalgia: the prototypical central sensitivity syndrome. Curr Rheumatol Rev. 2015;11(2):131-45. Available from: https://doi.org/10.2174/1573397111666150619095007
- Dombernowsky T, Dreyer L, Bartels EM, Danneskiold-Samsøe B. Muscular strength in patients with fibromyalgia. A literature review. Ugeskr Laeger. 2008;170(4):217-24. Available from: https://pubmed.ncbi.nlm.nih.gov/18282450/
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160-72. Available from: https://doi.org/10.1002/art.1780330203
- Kang J-H, Choi S-E, Park D-J, Lee SS. Disentangling Diagnosis and Management of Fibromyalgia. J Rheum Dis. 2022;29:4-13. Available from: https://doi.org/10.4078/jrd.2022.29.1.4
- Wolfe F, Clauw DJ, Fitzcharles M-A, Goldenberg DL, Häuser W, Katz RL, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016;46(3):319-329. Available from: https://doi.org/10.1016/j.semarthrit.2016.08.012
- Lee YH, Choi SJ, Ji JD, Song GG. Candidate gene studies of fibromyalgia: a systematic review and meta-analysis. Rheumatol Int. 2012;32(2):417-26. Available from: https://doi.org/10.1007/s00296-010-1678-9
- Behnoush AH, Khalaji A, Khanmohammadi S, Alehossein P, Saeedian B, Shobeiri P, et al. Brain-derived neurotrophic factor in fibromyalgia: A systematic review and meta-analysis of its role as a potential biomarker. PLoS One. 2023;18(12):e0296103. Available from: https://doi.org/10.1371/journal.pone.0296103
- Yunus MB. Genetic factors in fibromyalgia syndrome. Z Rheumatol. 1998;57 Suppl 2:61-2. Available from: https://doi.org/10.1007/s003930050237
- Rodriguez-Rodriguez L, Lamas JR, Abàsolo L, Baena S, Olano-Martin E, Collado A, et al. The rs3771863 single nucleotide polymorphism of the TACR1 gene is associated to a lower risk of sicca syndrome in fibromyalgia patients. Clin Exp Rheumatol. 2015;33(1 Suppl 88):S33-40. Available from: https://pubmed.ncbi.nlm.nih.gov/25786041/
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR®). APA; 2022. Available from: https://www.psychiatry.org/psychiatrists/practice/dsm
- Sommer C, Hauser W, Gerhold K, Joraschky P, Petzke F, Tölle T, Uçeyler N, et al. Etiology and pathophysiology of fibromyalgia syndrome and chronic widespread pain. Schmerz. 2008;22(3):267-82. Available from: https://doi.org/10.1007/s00482-012-1174-0
- Kudlow PA, Rosenblat JD, Weissman CR, Cha DS, Kakar R, McIntyre RS, Sharma V. Prevalence of fibromyalgia and co-morbid bipolar disorder: A systematic review and meta-analysis. J Affect Disord. 2015;188:134-42. Available from: https://doi.org/10.1016/j.jad.2015.08.030
- Emad Y, Regab Y, Zeinhom F, El-Khouly G, Abou-Zeid A, Rasker JJ. Hippocampus dysfunction may explain symptoms of fibromyalgia syndrome. A study with single-voxel magnetic resonance spectroscopy. J Rheumatol. 2008;35(7):1371-1377. Available from: https://pubmed.ncbi.nlm.nih.gov/18484688/
- Albrecht PJ, Hou Q, Argoff CE, Storey JR, Wymer JP, Rice FL. Excessive peptidergic sensory innervation of cutaneous arteriole-venule shunts (AVS) in the palmar glabrous skin of fibromyalgia patients: implications for widespread deep tissue pain and fatigue. Pain Med. 2013;6(14):895-915. Available from: https://doi.org/10.1111/pme.12139
- Simms RW, Goldenberg DL. Symptoms mimicking neurologic disorders in fibromyalgia syndrome. J Rheumatol. 1988;15(8):1271-1273. Available from: https://pubmed.ncbi.nlm.nih.gov/3184073/
- Park DC, Glass JM, Minear M, Crofford LJ. Cognitive function in fibromyalgia patients. Arthritis Rheum. 2001;44(9):2125-33. Available from: https://doi.org/10.1002/1529-0131(200109)44:9%3C2125::aid-art365%3E3.0.co;2-1
- Bangert AS, Glass JM, Welsh RC, Crofford LJ, Taylor SF, Park DC. Functional magnetic resonance imaging of working memory in fibromyalgia. Arthritis Rheum. 2003;48:S90. Available from: https://www.researchgate.net/publication/291785932_Functional_magnetic_resonance_imaging_of_working_memory_in_fibromyalgia
- Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27(15):4004-7. Available from: https://doi.org/10.1523/jneurosci.0098-07.2007
- Wood PB. Role of central dopamine in pain and analgesia. Expert Rev Neurother. 2008;8(5):781-97. Available from: https://doi.org/10.1586/14737175.8.5.781
- Orriols R, Costa R, Cuberas G, Jacas C, Castell J, Sunyer J. Brain dysfunction in multiple chemical sensitivity. J Neurol Sci. 2009;287(1–2):72–8. Available from: https://doi.org/10.1016/j.jns.2009.09.003
- Bascom R, Meggs WJ, Frampton M, Hudnell K, Killburn K, Kobal G, et al. Neurogenic inflammation: with additional discussion of central and perceptual integration of nonneurogenic inflammation. Environ Health Perspect. 1997;105 Suppl 2:531-7. Available from: https://doi.org/10.1289/ehp.97105s2531
- Link AS, Anikó K, Edvinsson L. Treatment of migraine attacks based on the interaction with the trigemino-cerebrovascular system. J Headache Pain. 2008;9(1):5-12. Available from: https://doi.org/10.1007/s10194-008-0011-4
- Weglicki WB, Phillips TM. Pathobiology of magnesium deficiency: a cytokine/neurogenic inflammation hypothesis. Am J Physiol. 1992;263(3 Pt 2):R734-7. Available from: https://doi.org/10.1152/ajpregu.1992.263.3.r734
- Devigili G, Tugnoli V, Penza P, Camozzi F, Lombardi R, Melli G, et al. The diagnostic criteria for small fiber neuropathy from symptoms of neuropathology. Brain. 2008;131(Pt 7):1912-25. Available from: https://doi.org/10.1093/brain/awn093
- Faber CG, Hoeijmakers JGJ, Ahn H-S, Cheng X, Han C, Choi JS, et al. Gain of function Naν1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol. 2012;71(1):26-39. Available from: https://doi.org/10.1002/ana.22485
- Rossi A, Di Lollo AC, Guzzo MP, Giacomelli C, Atzeni F, Bazzichi L, et al. Fibromyalgia and nutrition: what news? Clin Exp Rheumatol. 2015;33(1Suppl 88):S117–25. Available from: https://pubmed.ncbi.nlm.nih.gov/25786053/
- Caro XJ, Winter EF. The Role and Importance of Small Fiber Neuropathy in Fibromyalgia Pain. Curr Pain Headache Rep. 2015;19(12):55. Available from: https://doi.org/10.1007/s11916-015-0527-7
- Klein CJ, Lennon VA, Aston PA, McKeon A, Pittock SJ. Chronic pain as a manifestation of potassium channel-complex autoimmunity. Neurology. 2012;79(11):1136-44. Available from: https://doi.org/10.1212/wnl.0b013e3182698cab
- Sánchez-Domínguez B, Bullon P, Román-Malo L, Marín-Aguilar F, Alcocer-Gómez E, Carrión AM, et al. Oxidative stress, mitochondrial dysfunction and, inflammation common events in skin of patients with Fibromyalgia. Mitochondrion. 2015;21:69-75. Available from: https://doi.org/10.1016/j.mito.2015.01.010
- Isasi C, Colmenero I, Casco F, Tejerina E, Fernandez N, Serrano-Vela JI, et al. Fibromyalgia and non-celiac gluten sensitivity: a description with remission of fibromyalgia. Rheumatol Int. 2014;34(11):1607-12. Available from: https://doi.org/10.1007/s00296-014-2990-6
- Tzatha E, Chin RL. Small fiber abnormalities in skin biopsies of patients with benign fasciculations. J Clin Neuromuscul Dis. 2014;16(1):12-4. Available from: https://doi.org/10.1097/cnd.0000000000000047
- Minerbi A, Gonzalez E, Brereton NJ, Anjarkouchian A, Dewar K, Fitzcharles MA, et al. Altered microbiome composition in individuals with fibromyalgia. Pain. 2019;160(11):2589-2602. Available from: https://doi.org/10.1097/j.pain.0000000000001640
- Perrotta G. The intestinal microbiota: towards a multifactorial integrative model. Eubiosis and dysbiosis in morbid physical and psychological conditions. Arch Clin Gastroenterol. 2021;7(2):024-035. Available from: https://doi.org/10.17352/2455-2283.000094
- Perrotta G. Intestinal dysbiosis: definition, clinical implications, and proposed treatment protocol (Perrotta Protocol for Clinical Management of Intestinal Dysbiosis, PID) for the management and resolution of persistent or chronic dysbiosis. Arch Clin Gastroenterol. 2021;7(2):056-063. Available from: https://doi.org/10.17352/2455-2283.000100
- Perrotta G. Il microbiota intestinale nella pratica clinica. LK Ed.; 2022.
- O’Mahony LF, Srivastava A, Mehta P, Ciurtin C. Is fibromyalgia associated with a unique cytokine profile? A systematic review and meta-analysis. Rheumatology (Oxford). 2021;60(6):2602-2614. Available from: https://doi.org/10.1093/rheumatology/keab146
- Müller W, Schneider EM, Stratz T. The classification of fibromyalgia syndrome. Rheumatol Int. 2007;27(11):1005-10. Available from: https://doi.org/10.1007/s00296-007-0403-9
- Todesco S, Gambari PF, Punzi L. Malattie reumatiche. IV ed. McGraw-Hill Ed.; 2007.
- Barth H, Berg PA, Klein R. Is there any relationship between eosinophilia myalgia syndrome (EMS) and fibromyalgia syndrome (FMS)? An analysis of clinical and immunological data. Adv Exp Med Biol. 1999;467:487-96. Available from: https://doi.org/10.1007/978-1-4615-4709-9_61
- Wallace DJ, Wallace JB. All About Fibromyalgia: A Guide for Patients and their Families. SCC Print; 2002. Available from: https://books.google.com.bh/books?id=vco53badJAgC
- Rugarli C. Medicina interna sistematica. VIII Ed. Edra Ed.; 2021.
- Goldman L, Schafer AI. Goldman-Cecil: Medicina interna. XXV Ed. Edra Ed.; 2017.
- Goldenberg DL. Fibromyalgia: why such controversy? Ann Rheum Dis. 1995;54(1):3-5. Available from: https://doi.org/10.1136/ard.54.1.3
- Macfarlane GJ, Kronisch C, Dean LE, Atzeni F, Häuser W, Fluß E, et al. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis. 2017;76:318–328. Available from: https://doi.org/10.1136/annrheumdis-2016-209724
- Sarzi-Puttini P, Giorgi V, Marotta D, Atzeni F. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat Rev Rheumatol. 2020;16(11):645-660. Available from: https://doi.org/10.1038/s41584-020-00506-w
- Arnold B, Hauser W, Arnold M, Bernateck M, Bernardy K, Brückle W, et al. Multicomponent therapy of fibromyalgia syndrome. Systematic review, meta-analysis and guideline. Schmerz. 2012;26(3):287-90. Available from: https://doi.org/10.1007/s00482-012-1173-1
- Walitt B, Urrùtia G, Nishishinya MB, Cantrell SE, Häuser W. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev. 2015;2015(6):CD011735. Available from: https://doi.org/10.1002/14651858.cd011735
- Chinn S, Caldwell W, Gritsenko K. Fibromyalgia Pathogenesis and Treatment Options Update. Curr Pain Headache Rep. 2016;20(4):25. Available from: https://doi.org/10.1007/s11916-016-0556-x
- MacLean AJ, Schwartz TL. Tramadol for the treatment of fibromyalgia. Expert Rev Neurother. 2015;15(5):469-75. Available from: https://doi.org/10.1586/14737175.2015.1034693
- Carville SF, Arendt-Nielsen L, Bliddal H, Blotman F, Branco JC, Buskila D, et al. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis. 2008;67(4):536-41. Available from: https://doi.org/10.1136/ard.2007.071522
- Walitt B, Klose P, Phillips T, Phillips T, Häuser W. Antipsychotics for fibromyalgia in adults. Cochrane Database Syst Rev. 2016;2016(6):CD011804. Available from: https://doi.org/10.1002/14651858.cd011804.pub2
- Farag HM, Yunusa I, Goswami H, Sultan I, Doucette JA, Eguale T. Comparison of Amitriptyline and US Food and Drug Administration-Approved Treatments for Fibromyalgia: A Systematic Review and Network Meta-analysis. JAMA Netw Open. 2022;5(5):e2212939. Available from: https://doi.org/10.1001/jamanetworkopen.2022.12939
- Cameron EC, Hemingway SL. Cannabinoids for fibromyalgia pain: a critical review of recent studies (2015–2019). J Cannabis Res. 2020;2(1):19. Available from: https://doi.org/10.1186/s42238-020-00024-2
- Chaves C, Bittencourt PCT, Pelegrini A. Ingestion of a THC-Rich Cannabis Oil in People with Fibromyalgia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Pain Med. 2020;21(10):2212-2218. Available from: https://doi.org/10.1093/pm/pnaa303
- van Dam CJ, van Velzen M, Kramers C, Schellekens A, Olofsen E, Niesters M, et al. Cannabis-opioid interaction in the treatment of fibromyalgia pain: an open-label, proof of concept study with randomization between treatment groups: cannabis, oxycodone or cannabis/oxycodone combination-the SPIRAL study. Trials. 2023;24(1):64. Available from: https://doi.org/10.1186/s13063-023-07078-6
- Annunziato L, Di Rienzo G. Trattato di Farmacologia. Idelson-Gnocchi Ed.; 2020.
- Adler-Neal AL, Zeidan F. Mindfulness Meditation for Fibromyalgia: Mechanistic and Clinical Considerations. Curr Rheumatol Rep. 2017;19(9):59. Available from: https://doi.org/10.1007/s11926-017-0686-0
- Busch AJ, Schachter CL, Overend TJ, Peloso PM, Barber KA. Exercise for fibromyalgia: a systematic review. J Rheumatol. 2008;35(6):1130-44. Available from: https://pubmed.ncbi.nlm.nih.gov/18464301/
- Donaldson MS, Speight N, Loomis S. Fibromyalgia syndrome improved using a mostly raw vegetarian diet: an observational study. BMC Complement Altern Med. 2001;1:7. Available from: https://doi.org/10.1186/1472-6882-1-7
- Perrotta G. Omega-3/omega-6 fatty acids: The effects on the psychophysical well-being of adolescents and adults. Int J Clin Endocrinol Metab. 2023;9(1):008-018. Available from: https://doi.org/10.17352/ijcem.000057
- Del Giorno R, Skaper S, Paladini A, Varrassi G, Coaccioli S. Palmitoylethanolamide in Fibromyalgia: Results from Prospective and Retrospective Observational Studies. Pain Ther. 2015;4(2):169-78. Available from: https://doi.org/10.1007/s40122-015-0038-6
- Macian N, Dualé C, Voute M, Leray V, Courrent M, Bodé P, et al. Short-Term Magnesium Therapy Alleviates Moderate Stress in Patients with Fibromyalgia: A Randomized Double-Blind Clinical Trial. Nutrients. 2022;14(10):2088. Available from: https://doi.org/10.3390/nu14102088
- Aravena V, Garcìa PE, Tèllez A, Arias PR. Hypnotic intervention in people with fibromyalgia: A randomized controlled trial. Am J Clin Hypn. 2020;63(1):49-61. Available from: https://doi.org/10.1080/00029157.2020.1742088
- Qu K, Li M-X, Zhiu Y-L, Yu P, Dong M. The efficacy of vitamin D in treatment of fibromyalgia: a meta-analysis of randomized controlled studies and systematic review. Expert Rev Clin Pharmacol. 2022;15(4):433-442. Available from: https://doi.org/10.1080/17512433.2022.2081151
- Cardona D, Roman P, Canadas F, Sánchez-Labraca N. The Effect of Multiprobiotics on Memory and Attention in Fibromyalgia: A Pilot Randomized Controlled Trial. Int J Environ Res Public Health. 2021;18(7):3543. Available from: https://doi.org/10.3390/ijerph18073543
- Ghavidel-Parsa B, Naeimi A, Gharibpoor F, Sattari N, Jafari A, Masooleh IS, et al. Effect of vitamin B6 on pain, disease severity, and psychological profile of fibromyalgia patients; a randomized, double-blinded clinical trial. BMC Musculoskelet Disord. 2022;23(1):664. Available from: https://doi.org/10.1186/s12891-022-05637-7
- Tirelli U, Pavanello M, Piasentin C, Piasentin C, Lleshi A, Taibi R. Ozone therapy in 65 patients with fibromyalgia: an effective therapy. Eur Rev Med Pharmacol Sci. 2019;23(4):1786-1788. Available from: https://doi.org/10.26355/eurrev_201902_17141
- Lloyd DM, Wittkopf PG, Arendsen LJ, Jones AKP. Is Transcranial Direct Current Stimulation (tDCS) Effective for the Treatment of Pain in Fibromyalgia? A Systematic Review and Meta-Analysis. J Pain. 2020;21(11-12):1085-1100. Available from: https://doi.org/10.1016/j.jpain.2020.01.003
- Klemm P, Becker J, Aykara I, Asendorf T, Dischereit G, Neumann E, et al. Serial whole-body cryotherapy in fibromyalgia is effective and alters cytokine profiles. Adv Rheumatol. 2021;61(1):3. Available from: https://doi.org/10.1186/s42358-020-00159-z
- Yeh S-W, Hong C-H, Shih M-C, Tam KW, Huang YH, Kuan YC. Low-Level Laser Therapy for Fibromyalgia: A Systematic Review and Meta-Analysis. Pain Physician. 2019;22(3):241-254. Available from: https://pubmed.ncbi.nlm.nih.gov/31151332/
- Kundakci B, Kaur J, Goh SL, Hall M, Doherty M, Zhang W, et al. Efficacy of nonpharmacological interventions for individual features of fibromyalgia: a systematic review and meta-analysis of randomised controlled trials. Pain. 2022;163(8):1432-1445. Available from: https://doi.org/10.1097/j.pain.0000000000002500
- Serrat M, Sanabria-Mazo JP, Almirall M, Musté M, Feliu-Soler A, Méndez-Ulrich JL, et al. Effectiveness of a Multicomponent Treatment Based on Pain Neuroscience Education, Therapeutic Exercise, Cognitive Behavioral Therapy, and Mindfulness in Patients With Fibromyalgia (FIBROWALK Study): A Randomized Controlled Trial. Phys Ther. 2021;101(12):pzab200. Available from: https://doi.org/10.1093/ptj/pzab200
- Menten LA, Franco KFM, Franco YRS, Miyamoto GC, Reis FJJ, Cabral CMN. Do patients with fibromyalgia have body image and tactile acuity distortion? Pain Pract. 2022;22(8):678-687. Available from: https://doi.org/10.1111/papr.13153
- Perrotta G. The concept of altered perception in "body dysmorphic disorder": the subtle border between the abuse of selfies in social networks and cosmetic surgery, between socially accepted dysfunctionality and the pathological condition. J Neurol Neurol Sci Disord. 2020;6(1):001-007. Available from: http://dx.doi.org/10.17352/jnnsd.000036
- Kaleycheva N, Cullen AE, Evans R, Harris T, Nicholson T, Chalder T. The role of lifetime stressors in adult fibromyalgia: systematic review and meta-analysis of case-control studies. Psychol Med. 2021;51(2):177-193. Available from: https://doi.org/10.1017/s0033291720004547
- Migliorini F, Maffulli N, Eschweiler J, Betsch M, Tingart M, Colarossi G. Placebo effect in pharmacological management of fibromyalgia: a meta-analysis. Br Med Bull. 2021;139(1):73-85. Available from: https://doi.org/10.1093/bmb/ldab015
- Mitsikostas DD, Chalarakis NG, Mantonakis LI, Delicha EM, Sfikakis PP. Nocebo in fibromyalgia: meta-analysis of placebo-controlled clinical trials and implications for practice. Eur J Neurol. 2012;19(5):672-80. Available from: https://doi.org/10.1111/j.1468-1331.2011.03528.x
- Hauser W, Sarzi-Puttini P, Tolle TR, Wolfe F. Placebo and nocebo responses in randomised controlled trials of drugs applying for approval for fibromyalgia syndrome treatment: systematic review and meta-analysis. Clin Exp Rheumatol. 2012;30(6 Suppl 74):78-87. Available from: https://pubmed.ncbi.nlm.nih.gov/23137770/
- Boehm K, Raak C, Cramer H, Lauche R, Ostermann T. Homeopathy in the treatment of fibromyalgia--a comprehensive literature-review and meta-analysis. Complement Ther Med. 2014;22(4):731-42. Available from: https://doi.org/10.1016/j.ctim.2014.06.005
- Langhorst J, Hauser W, Bernardy K, Lucius H, Settan M, Winkelmann A, et al. Complementary and alternative therapies for fibromyalgia syndrome. Systematic review, meta-analysis and guideline. Schmerz. 2012;26(3):311-7. Available from: https://doi.org/10.1007/s00482-012-1178-9
- Deare JC, Zheng Z, Xue CCL, Liu JP, Shang J, Scott SW, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;2013(5):CD007070. Available from: https://doi.org/10.1002/14651858.cd007070.pub2
- Mist SD, Jones KD. Randomized Controlled Trial of Acupuncture for Women with Fibromyalgia: Group Acupuncture with Traditional Chinese Medicine Diagnosis-Based Point Selection. Pain Med. 2018;19(9):1862-1871. Available from: https://doi.org/10.1093/pm/pnx322
- Valera-Calero JA, Fernàndez-de-Las-Penas C, Navarro-Santana MJ, et al. Efficacy of Dry Needling and Acupuncture in Patients with Fibromyalgia: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2022;19(16):9904. Available from: https://doi.org/10.3390/ijerph19169904
- Sotte L. Fondamenti di agopuntura e medicina cinese. CEA Ed.; 2007.
- Sotte L. Farmacologia cinese. CEA Ed.; 2010.
- Di Concetto G, Sotte L. Agopuntura cinese. CEA Ed.; 2007.
- Cao H, Liu J, Lewith GT. Traditional Chinese Medicine for treatment of fibromyalgia: a systematic review of randomized controlled trials. J Altern Complement Med. 2010;16(4):397-409. Available from: https://doi.org/10.1089/acm.2009.0599
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
