Archives of Otolaryngology and Rhinology
Between Shadow and Light: The Experience of the Fann National University Hospital in Treating Maxillary Sinus cancers (Dakar, Senegal)
ENT Department, Fann University Hospital, Dakar, Senegal
Author and article information
Cite this as
Fall F, et al. Between Shadow and Light: The Experience of the Fann National University Hospital in Treating Maxillary Sinus cancers (Dakar, Senegal). Arch Otolaryngol Rhinold. 2025; 11(3): 026-030. Available from: 10.17352/2455-1759.000163
Copyright License
© 2025 Fall F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction: Maxillary sinus cancers are a rare and complex pathological entity, often overlooked due to their low incidence and specific anatomical location. The study aimed to report epidemiological, clinical, and paraclinical data and to evaluate the results of treatment.
Methods: This is a retrospective, analytical, and descriptive study analyzing 79 cases of maxillary sinus cancer hospitalized and treated in the ENT department over 7 years, from January 2017 to December 2023.
Results: The mean age of our patients was 49 years, and the sex ratio (M/F) was 1.27. The predominant symptoms included nasal obstruction (70%) and paranasal or palatal swelling, while advanced forms presented with ophthalmological signs in 22.22% of cases. A scan was performed in 69 patients (87.3%). The diagnostic certainty remains anatomopathological. Thus, 50.6% of cases were squamous cell carcinomas, followed by 16.5% adenocarcinomas. In terms of treatment, 45 patients underwent ipsilateral maxillectomy with reconstruction, supplemented by adjuvant chemoradiotherapy in 37 patients and radiotherapy alone in 8 others. Overall survival was 56%, with two cases of tumor progression and one case of recurrence.
Overall survival was 56%. The cancer progression was marked by continued progression in two patients and one case of tumor recurrence.
Conclusion: Maxillary sinus cancers, although rare, represent a diagnostic and therapeutic challenge due to their anatomical and clinical complexity. A multidisciplinary approach and increased vigilance are essential to optimize their management and improve patient prognosis.
Maxillary sinus cancers are relatively rare tumors, accounting for only 3 to 4% of upper aerodigestive tract cancers and approximately 1% of all cancers, with the sex ratio (M/F) 2 [1-3]. The incidence of paranasal sinus tumors is 0.2-0.8%, with 60% of these tumors originating in the maxillary sinus. Squamous cell carcinoma (SCC) of the maxillary sinus accounts for 1% of all malignant tumors. The development in confined anatomical regions, nonspecific clinical signs, and the absence of risk factors often lead to late treatment of lesions, for which the prognosis remains poor. For locally advanced forms, the standard treatment combines surgery and radiotherapy, with chemotherapy generally reserved for inoperable patients. Through a review of the literature, we will share our experience with 79 cases collected over seven years.
Patients and methods
This was a retrospective study involving 79 patients managed in the Otorhinolaryngology Department of the Fann National University Hospital Center from January 1, 2017, to December 31, 2023. All patients diagnosed with maxillary sinus cancer confirmed by histopathological examination were included. Patients whose tumors originated in the nasal cavity, ethmoid, sphenoid, and nasopharynx were excluded from the study.
The study was conducted using information from patients' medical records and imaging examinations. The elements analyzed included age, sex, personal history, occupation, symptoms presented, and their duration before diagnosis. We also examined clinical manifestations, tumor extent confirmed by imaging studies, and histological characteristics of the lesion. Finally, treatment modalities were detailed, including the type of surgery performed, the quality of resection as assessed by pathology, postoperative complications, and the administration of radiotherapy or chemotherapy, if any.
Results
The study included 79 patients, comprising 47 men and 32 women, with a male-to-female sex ratio of 1.27. The average age at diagnosis was 49, with the most represented age group being between 40 and 50. In terms of medical and surgical history, dental problems (either chronic toothache or tooth extraction) were the most common risk factor, with 11 of our patients having five cases of smoking, two cases of alcoholism, and one case of exposure to wood dust. Forty-seven percent of patients had no known risk factors.
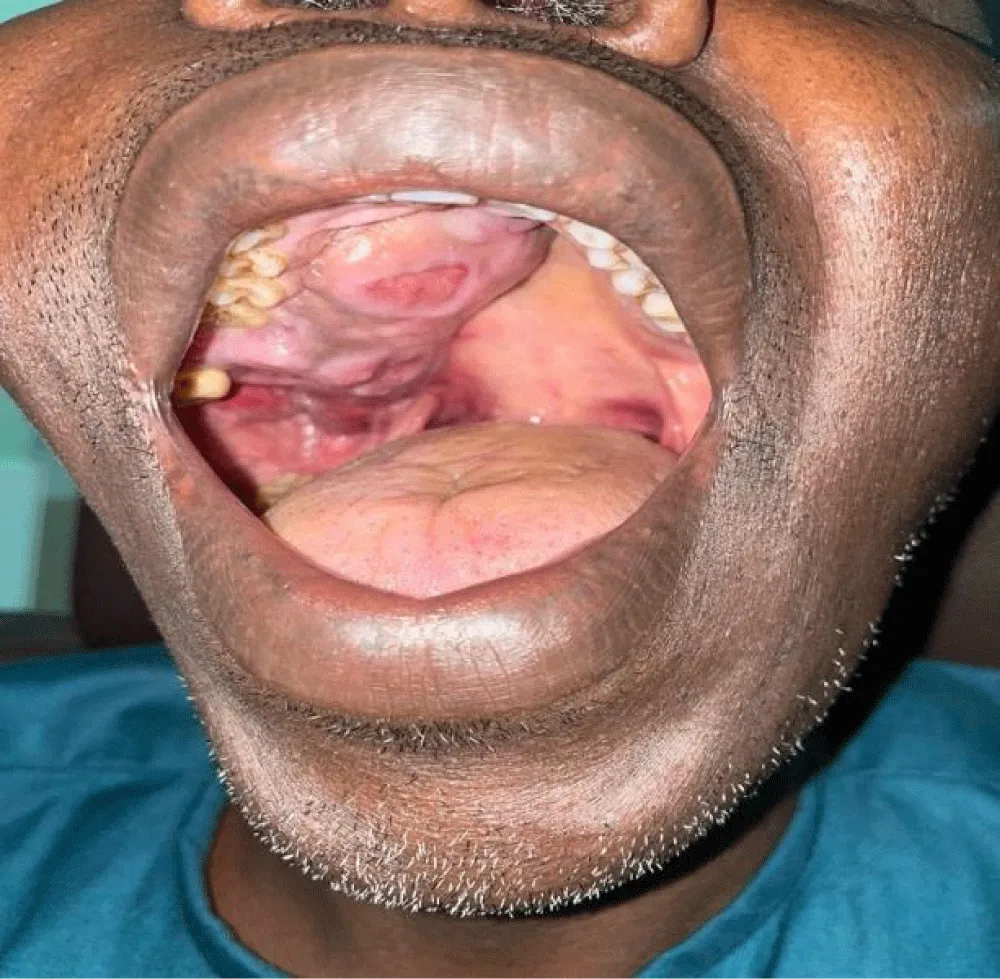
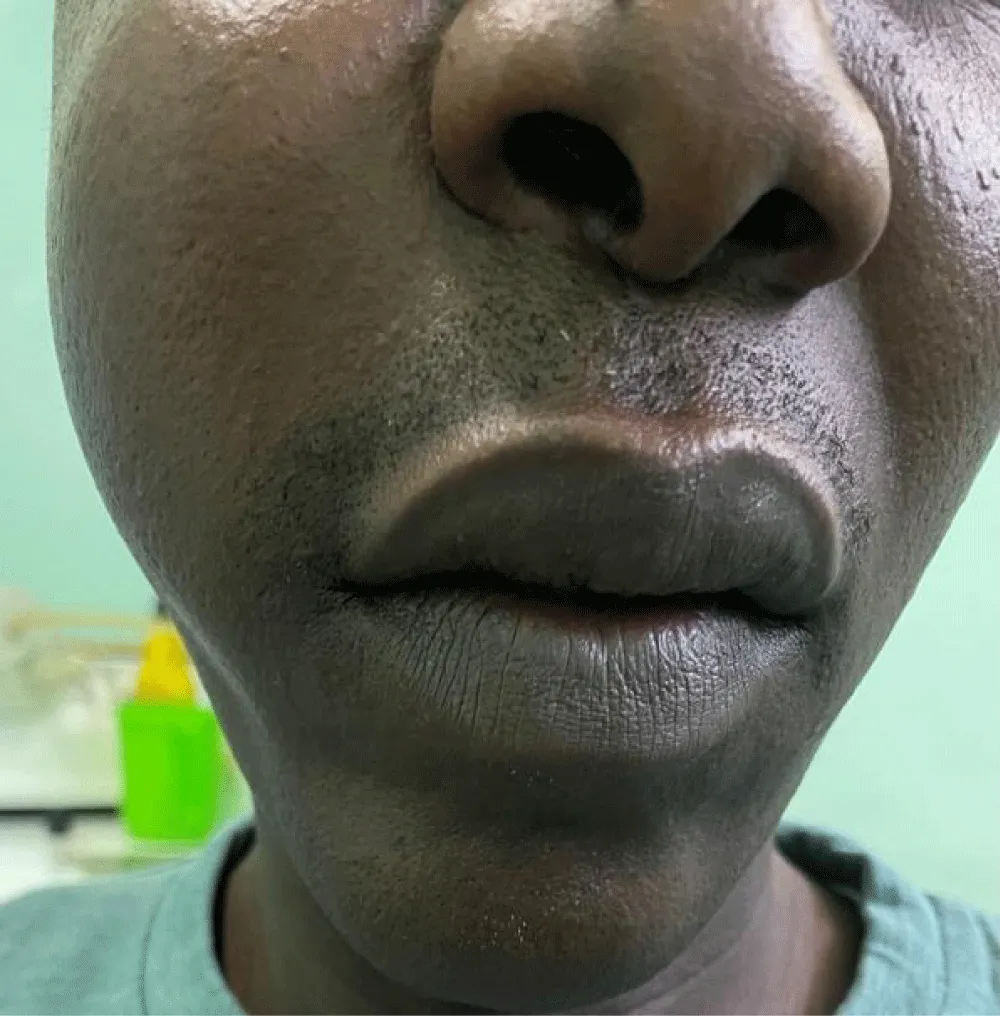
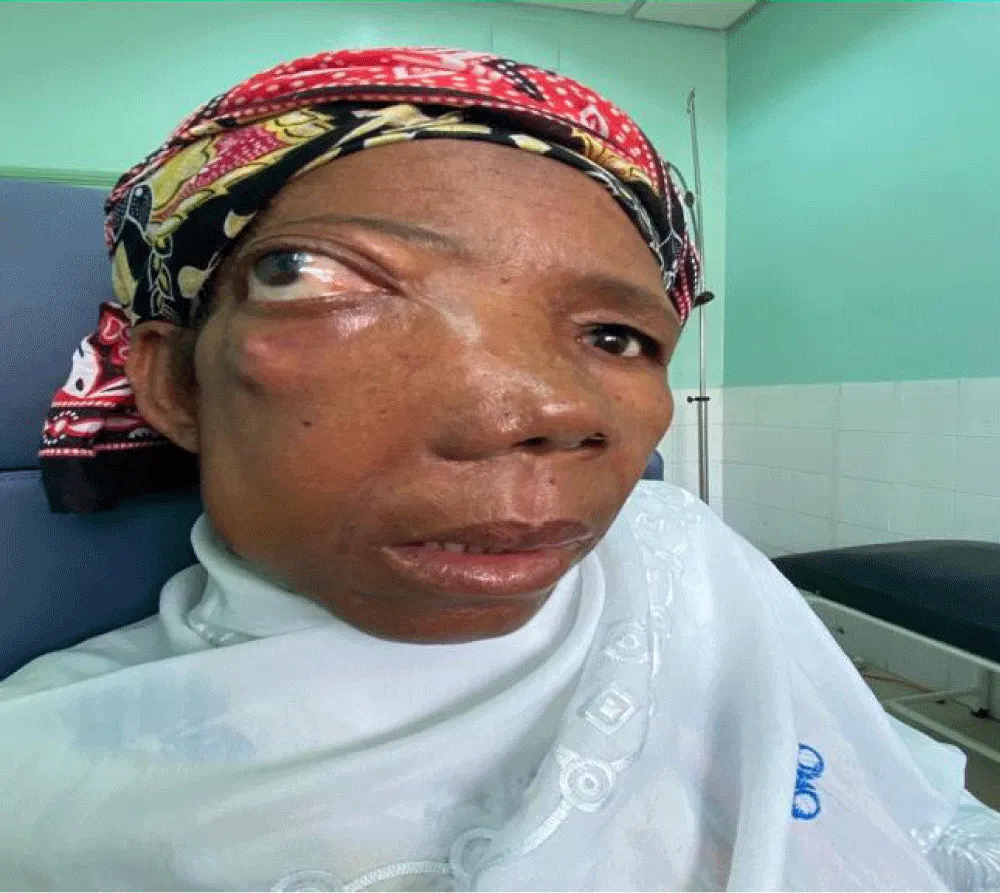
The average duration of symptoms before diagnosis was 15 months. The most common clinical signs were nasal obstruction (70%), followed by palatal swelling (Figure 1) or cheek swelling (63%) (Figure 2), an endonasal mass (48%), and epistaxis (41.7%). In advanced cases, ophthalmological signs such as exophthalmos or decreased visual acuity were observed in 22.22% of cases (Figure 3). At admission, 70% of patients had no palpable lymphadenopathy.
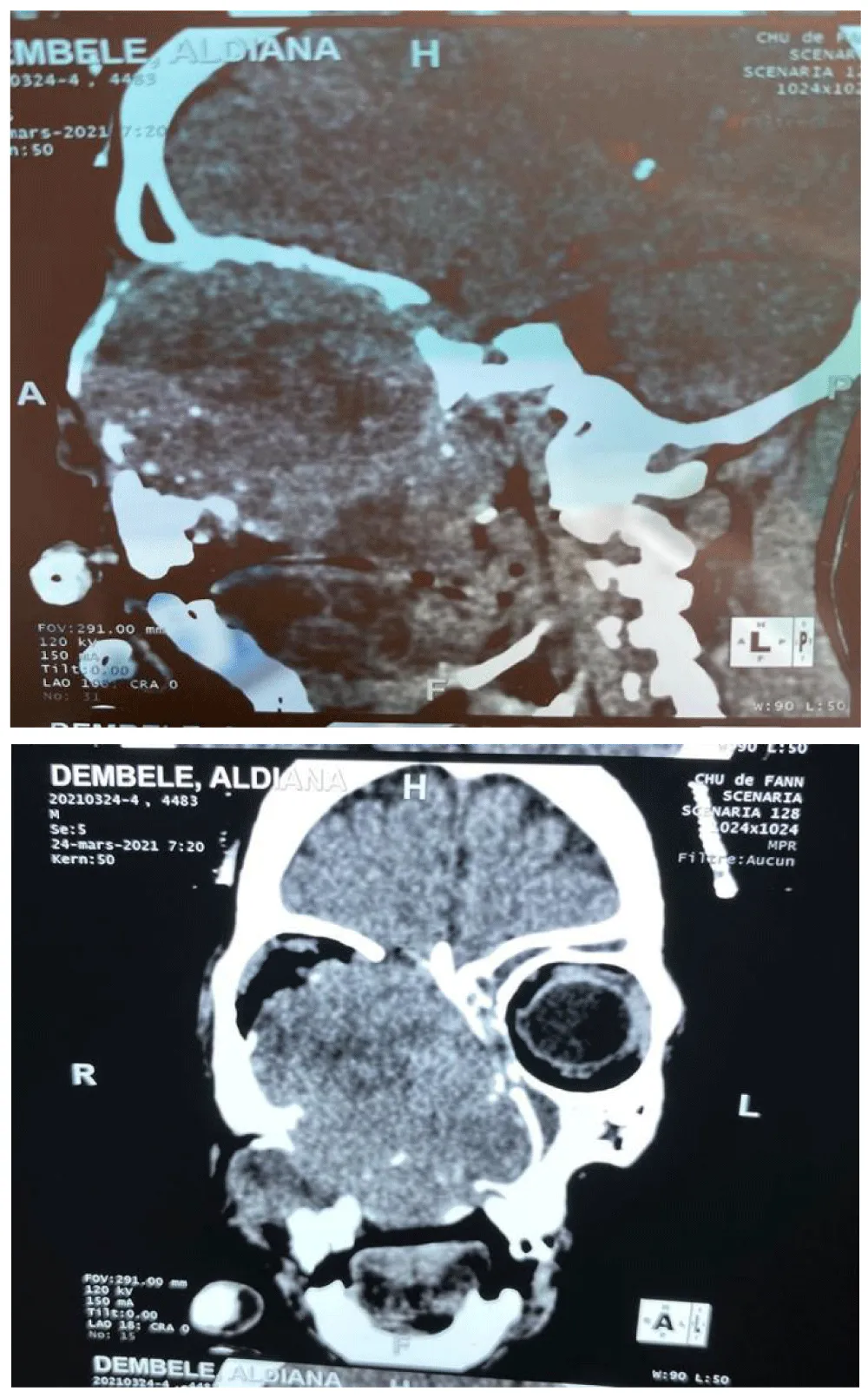
Computed tomography (CT) was performed in 87.3% of patients (n=69) (Figure 4), and two patients underwent MRI, allowing visualization of the tumor and assessment of its locoregional and distant spread. The lesions generally presented as a solid, homogeneous tumor mass, enhancing after contrast injection, often osteolytic, and occupying the maxillary sinus. Data analysis showed that epithelial tumors accounted for 81% of cases and non-epithelial tumors for 19% of cases, of which 50.6% were squamous cell carcinomas and 16.5% were adenocarcinomas.
The clinical examination data and imaging results were used to classify the tumor according to the UICC international classification.
Of the tumors, 70.8% were classified as T4 (n=56), 24% as T3, and 5% as T2.
Nineteen percent of patients with metastatic lymph node involvement were classified as N1,
11% as N2, and 94% as M0, while 5% had metastases.
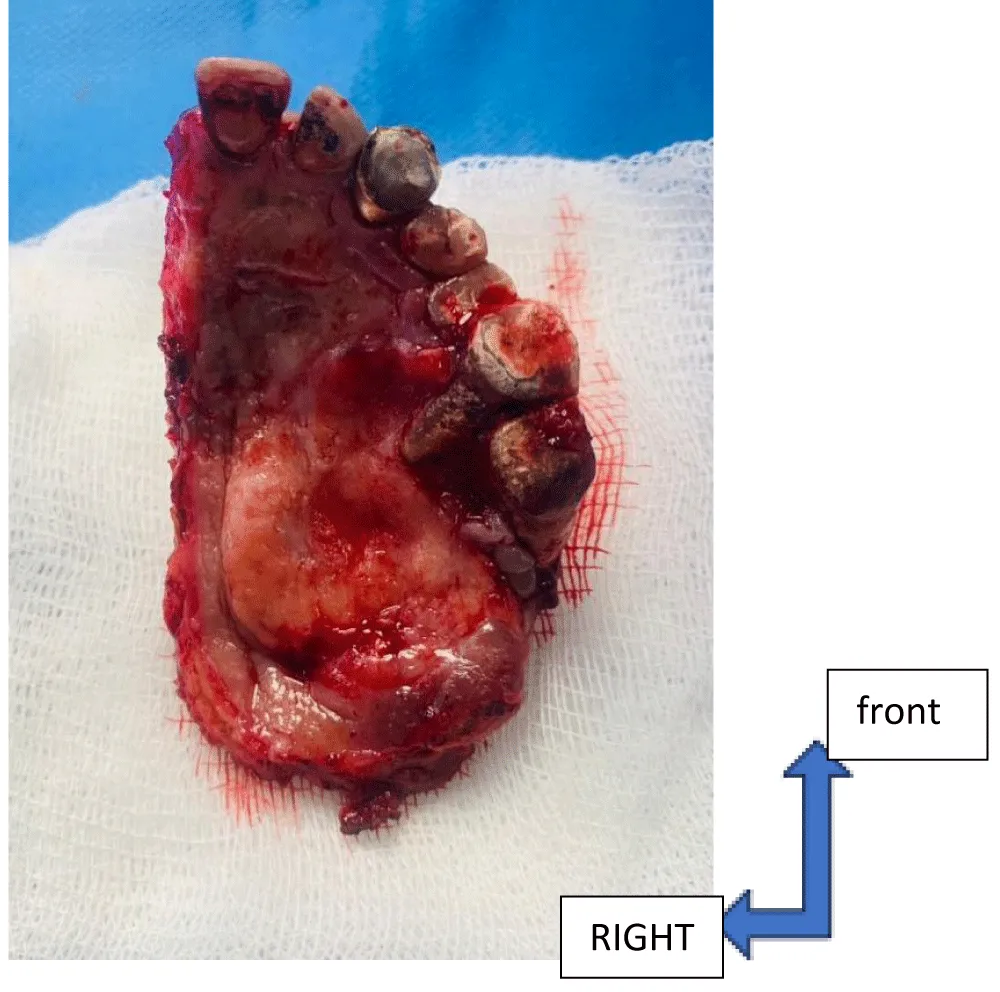
With a view to cure, 57% of patients underwent maxillectomy (Figure 5) and, of these, 27% underwent prosthetic reconstruction. The indications were determined during a multidisciplinary consultation meeting. Surgery was supplemented by adjuvant chemoradiotherapy in 46% of patients and by radiotherapy alone in 10% of patients. Chemoradiotherapy alone was also administered to 23% of patients.
Overall survival was 56%. The cancer progressed in two patients and recurred in one case. After one year of follow-up, mortality was 10.1%.
Discussion
Malignant tumours of the nasal cavity and paranasal sinuses are rarely found, with the maxillary sinus being the most frequently affected subsite, representing approximately 80% of malignant tumours in this area [5,6]. In this retrospective study conducted over a period of 7 years, maxillary sinus cancer was more frequent in men, with a male-to-female ratio of 1.7 and a mean age of 50 years [7-9]. Recognized carcinogens include tobacco, human papillomavirus (HPV) infection, industrial toxic agents (glues, nickel, formaldehyde, synthetic leather, chromium, and others), and exposure to textile dust in women [1,10].
Our patients consulted within an average of 15 months after the onset of their symptoms. Patients, therefore, seem to consult after several months of progression, which may be due to the neglect of certain symptoms. Added to this is the slow progression of symptoms and difficulties in accessing care for some patients. As a result, cancer is generally diagnosed at an advanced stage[7,11]. Symptoms are dominated by nasal obstruction followed by jugal or palatal swelling, while epistaxis rarely exceeds 50%. The most suggestive criterion is the unilateral nature of these symptoms.
Regarding histological distribution, the results of this series are consistent with the data in the literature: epithelial tumors predominate [7,12], with a predominance of squamous cell carcinomas, representing 50% of cases, followed by adenoid cystic carcinomas [13,14]. Advanced locoregional spread, confirmed by CT imaging, which is the key diagnostic test, is often present at the time of initial diagnosis, despite a short interval between the onset of the first symptoms and consultation. This rapid progression can be explained by the low specificity and late onset of symptoms in maxillary sinus cancer [4]. This allowed us to classify our patients according to the TNM classification. Consequently, 71% of our patients presented at an advanced stage classified as T4, and 24% were classified as T3 [15]. At the time of diagnosis, 70–80% of maxillary sinus cancers show local extension [16] and are classified as T3 or T4 according to the American Joint Committee on Cancer staging system [17]. Unlike other malignant tumors of the head and neck, where the incidence of cervical lymph node metastasis is high, global data from the literature indicate that lymph node metastases are rare in sinonasal malignancies [2,9]. Our study reported similar findings, with seventy percent of patients being lymph node–negative. Despite the low incidence of cervical lymph node metastasis in maxillary sinus squamous cell carcinoma, which is generally managed conservatively, Scurry et al. reported regional recurrence rates of up to 18% and emphasized the need for elective neck dissection in N0 necks [27].
According to the TNM classification, 70% of our patients were classified as N0, and 30% had lymph node involvement. Lymph node metastasis is not commonly seen in maxillary sinus squamous cell carcinoma, with reported incidences ranging from 3.3% to 26% [2,9].
The literature indicates that surgery followed by adjuvant radiotherapy is the most commonly used treatment modality for maxillary sinus squamous cell carcinoma (SCC) [18]. Indeed, complete resections are rare. It has been shown that, in general, the rate of confirmed incomplete resection reached 48% and that margins were uncertain in 25% of patients [2].
The most commonly used surgical techniques are currently endoscopic endonasal techniques, sometimes assisted by robots [19,20]. Endonasal surgery has long been reserved for small tumors or nasoethmoidal tumors, but improvements in instrumentation and neuronavigation now allow for the resection of much larger tumors.
However, in our series, the endoscopic endonasal approach was not used. Over the last three decades, the indications for endoscopic surgery for maxillary sinus cancers have rarely been manageable with a purely endoscopic approach and have therefore received less attention in the contemporary literature.
Maxillectomy was performed in 56.9% of cases, exclusively via an external approach. This can be explained, on the one hand, by the fact that most of our patients were classified as T4 according to TNM, and, on the other hand, because in our context, endoscopic surgery is not used for curative purposes for maxillary sinus cancers due to the extent of the tumor. This therapeutic approach is shared in many series [8,21,22]. Surgical treatment is more beneficial than chemotherapy [23].
All authors agree that the combination of primary surgery followed by postoperative radiotherapy provides the best local control [19].
However, the literature shows that adjuvant postoperative radiotherapy or chemotherapy is associated with better survival outcomes in malignant tumors of the maxillary sinus than surgery alone [23,24]. Kuo and colleagues reported that the overall survival rate of patients with maxillary sinus squamous cell carcinoma improved with neoadjuvant treatment [25]. Adjuvant chemoradiotherapy plays an important role in the treatment of maxillary sinus tumors with positive margins and undesirable characteristics [24,26]. Our study found no significant difference in survival outcomes based on treatment modalities.
These cancers are known to be highly recurrent [2,9,27]. This is because tumor margins are often difficult to assess, with sometimes extensive locoregional spread of the tumor. Added to this are difficulties in accessing complementary treatment for some patients, advanced stage at diagnosis, and poor post-treatment follow-up. Thus, in our series, 3.79% of recurrences were noted. We consider this figure to be an underestimate, taking into account patients lost to follow-up and the follow-up period. According to a study conducted at the CHU/FANN (Senegal 2018) [28], 48.71% of recurrences were found.
In the literature, the overall 5-year survival rate for SCC of the maxillary sinus has been reported to be 25-50% [8], and our study showed similar results with a 5-year survival rate of up to 33%. These data suggest that maxillary sinus cancer remains an area of HN oncology that deserves to be better understood and explored.
Conclusion
The experience of the CHNU de Fann in the management of maxillary sinus cancers illustrates both the immense challenges and significant advances in the field of public health in Senegal. Between light and shadow, this struggle highlights persistent obstacles, such as late diagnosis, limited resources, and inequalities in access to care. However, it also reveals the resilience of healthcare professionals, their unwavering commitment, and the progress made through innovative approaches and multidisciplinary collaboration.
These cancers, which are often complex and devastating, require a comprehensive response that integrates prevention, early diagnosis, appropriate treatment, and psychosocial support. The Fann University Hospital Center, as a pillar in the fight against these diseases, embodies this desire to turn challenges into opportunities. Nevertheless, to strengthen this fight, increased institutional support, greater public awareness, and improved access to specialized care remain essential.
Author contributions
All authors have read and approved the final version of the manuscript.
- Guillemin F, Blanchard P, Boisselier P, Brahimi Y, Calugaru V, Coutte A, et al., Proposition de délinéation des volumes cibles anatomocliniques postopératoires de la tumeur primitive des cancers du sinus maxillaire et des cavités nasales, Cancer/Radiothérapie. 2024;(28)1:218-227. Available from: https://doi.org/10.1016/j.canrad.2023.12.001
- Anjum W, Maken RN, Nisar H, Fatima I, Masood M, Shahid AB. Epidemiology and treatment outcomes of sinonasal tumors: a single institute’s experience in Pakistan. J Coll Physicians Surg Pak. 2019;29:356-60. https://doi.org/10.29271/jcpsp.2019.04.356
- Sylvester MJ, Fenberg R, McKean EL, VanKoevering KK. Treatment of sinonasal squamous cell carcinoma: the experience at a single tertiary care facility over 32 years. J Neurol Surg B Skull Base. 2020;81:021. Available from: https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0040-1702311
- Galloni C, Locatello LG, Bruno C, Cannavicci A, Maggiore G, Gallo O. The role of elective neck treatment in the management of sinonasal carcinomas: a systematic review of the literature and a meta-analysis. Cancers (Basel). 2021;13:10.3390/cancers13081842.
- Kılıç S, Kılıç SS, Baredes S, Park RCW, Mahmoud O. Comparison of endoscopic and open resection of sinonasal squamous cell carcinoma: A propensity score-matched analysis of 652 patients. Int. Forum Allergy Rhinol. 2018;(8):421–434. Available from: https://doi.org/10.1002/alr.22040
- Ferrari M, Ioppi A, Schreiber A, Gualtieri T, Mattavelli D, Rampinelli V, et al., Stefano Taboni, Michele Tomasoni, Paolo Bossi, Alberto Deganello, Piero Nicolai, Malignant tumors of the maxillary sinus: Prognostic impact of neurovascular invasion in a series of 138 patients, Oral Oncology. 2020;(106):104672, ISSN 1368-8375. Available from:https://doi.org/10.1016/j.oraloncology.2020.104672
- Nachtsheim L, Möller L, Oesterling F, Kajueter H, Stang A, Hieggelke L, et al. Cancer of the paranasal sinuses in Germany: data on incidence and survival from a population-based cancer registry. Cancer Epidemiol. 2024;93:102684. Available from: https://doi.org/10.1016/j.canep.2024.102684
- Dhanani R, Faisal M, Shahid H, Malik KI, Jamshed A, Hussain R. Outcomes of management of sinonasal malignancies at a dedicated cancer institution: a retrospective study. Ann Maxillofac Surg. 2021;11:115-20. Available from: https://doi.org/10.4103/ams.ams_16_21
- 4. Cancers rhinosinusiens.
- Castelnuovo Turri-Zanoni MP, Battaglia P, Antognoni P, Bossi P, Locatelli D. Sinonasal malignancies of anterior skull base: histology-driven treatment strategies Otolaryngol Clin North Am. 2016. Available from: https://doi.org/10.1016/j.otc.2015.09.012
- Deganello Ferrari MA, Paderno A, Turri-Zanoni M, Schreiber A, Mattavelli D, Endoscopic-assisted maxillectomy: operative technique and control of surgical margins. Oral Oncol. 2019. Available from: https://doi.org/10.1016/j.oraloncology.2019.04.002
- Husain Q, Joshi RR, Cracchiolo JR, Roman BR, Ganly I, Tabar V, et al., Cohen, M.A. Surgical Management Patterns of Sinonasal Malignancy: A Population-Based Study. J. Neurol. Surg. Part B Skull Base 2018;(80):371–379. Available from: https://doi.org/10.1055/s-0038-1675233
- Dutta R, Ba PMD, Svider PF, Liu JK, Baredes S, Eloy JA. Sinonasal malignancies: A population-based analysis of site-specific incidence and survival. Laryngoscope 2015 ;(125):2491–2497. Available from: https://doi.org/10.1002/lary.25465
- Gaye PM, Dieng MM, Kasse AA, Diouf D, Dem A, Diop M. Carcinome épidermoïde du sinus maxillaire: étude rétrospective de 30 cas de chimiothérapie néoadjuvante. J Afr Cancer Afr J Cancer. 1 août 2010;2(3):156‑9. Available from: https://link.springer.com/article/10.1007/s12558-010-0097-x
- Chang HJ, Hur J, Won KY, Chang B, Lee HY. "Recurrent maxillary sinus cancer with only adrenal metastasis". Molecular and Clinical Oncology. 2017;(7)5:847-850. Available from: https://doi.org/10.3892/mco.2017.1427.
- Keerio AA, Qayyum MU, Kashif A, Dhanani R, Rashid A, Faisal M, et al. Treatment Outcomes of Maxillary Sinus Squamous Cell Carcinoma at a Dedicated Cancer Institute: A Retrospective Study. Cureus 2022;14(6): e25644. Available from: https://doi.org/10.7759/cureus.25644
- Dooley L, Shah J: Management of the neck in maxillary sinus carcinomas. Curr Opin Otolaryngol Head Neck Surg. 2025;23:107-114.PubMed/NCBI View Article : Google Scholar. Available from: https://doi.org/10.1097/moo.0000000000000138
- Shen W, Sakamoto N, Yang L. Prognostic models and nomograms for predicting survival of patients with maxillary sinus carcinomas. Int Forum Allergy Rhinol. 2017;(7):741-748. 10.1002/alr 21950. Available from: https://doi.org/10.1002/alr.21950
- Colevas AD, Yom SS, Pfister DG, Spencer S, Adelstein D, Adkins D, NCCN Guidelines Insights: Head and Neck Cancers, Version 1.2018. J Natl Compr Canc Netw. 2018;(16):479-490. Available from: https://doi.org/10.6004/jnccn.2018.0026
- Grégoire V, Evans M, Le QT, Bourhis J, Budach V, Chen A, at al., Delineation of the primary tumour clinical target volumes (CTV-P) in laryngeal, hypopharyngeal, oropharyngeal and oral cavity squamous cell carcinoma: AIRO, CACA, DAHANCA, EORTC, GEORCC, GORTEC, HKNPCSG, HNCIG, IAG-KHT, LPRHHT, NCIC CTG, NCRI, NRG Oncology, PHNS, SBRT, SOMERA, SRO, SSHNO, TROG consensus guidelines Radiother Oncol. 2018. Available from: https://doi.org/10.1016/j.radonc.2017.10.016
- Darouassi Y, Touati MM, Chihani M, Alami JE, Bouaity B, Aamar H. Les tumeurs malignes naso-sinusiennes: à propos de 32 cas et revue de la littérature. Pan Afr Med J [en ligne]. 2015 [cité le 8 décembre 2024];22. Disponible sur: Available from: http://www.panafrican-med-journal.com/content/article/22/342/full/.
- Taylor MA, Saba NF. Cancer of the paranasal sinuses. Hematol Oncol Clin North Am. 2021;35(5):949-962. Available from: https://doi.org/10.1016/j.hoc.2021.05.006
- Wang Y, Yang R, Zhao M, Guo W, Zhang L, Zhang W, et al. Retrospective analysis of 98 cases of maxillary sinus squamous cell carcinoma and therapeutic exploration. World J Surg Oncol. 2020;18:90. Available from: https://doi.org/10.1186/s12957-020-01862-3
- Qiu X, Yang J. Clinical study of cetuximab combined with radical radiotherapy in the treatment of locally advanced sinonasal squamous cell carcinoma. J BUON. 2018;23:1111-1117. Available from: https://pubmed.ncbi.nlm.nih.gov/30358219/
- Kuo P, Torabi SJ, Kraus D, Judson BL. Survival outcomes for induction vs adjuvant chemotherapy in squamous cell carcinoma of the maxillary sinus. Otolaryngology-Head and Neck Surgery. 2019;(160):658-663. Available from: https://doi.org/10.1177/0194599818804777
- Ebara T, Ando K, Eishima J, Suzuki M, Kawakami T, Horikoshi H, et al. Radiation with concomitant superselective intra-arterial cisplatin infusion for maxillary sinus squamous cell carcinoma. Jpn J Radiol. 2019;(37):494-499. Available from: https://doi.org/10.1007/s11604-019-00827-1
- Khan Z. Clinical signs and symptoms of malignant tumors involving maxillary sinus: recommendation of an examination sieve and risk alarm score. Int J Oral Maxillofac Surg. 2024;52:98-99. Available from: https://www.ijoms.com/article/S0901-5027(23)00567-2/abstract
- Barry W, les tumeurs malignes naso-sinusiennes :à propos de 39 cas colligés à la clinique ORL Lamine Sine Diop ,thèse de doctorat, université Cheikh Anta Diop de Dakar, 2018.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
