Archives of Otolaryngology and Rhinology
Epidermal Growth Factor Receptor (EGFR) and Vascular Endothelial Growth Factor (VEGF) expression in Sinonasal Inverted Papillomas
Mario Bilic1*, Ena Klaric1, Lana Kovac-Bilic1, Selma Hodzic-Redzic1 and Sven Seiwerth2
2Department of Pathology, University Hospital Center Zagreb, Zagreb, Croatia
Cite this as
Bilic M, Klaric E, Bilic LK, Redzic SH, Seiwerth S (2018) Epidermal Growth Factor Receptor (EGFR) and Vascular Endothelial Growth Factor (VEGF) expression in Sinonasal Inverted Papillomas. Arch Otolaryngol Rhinol 4(4): 101-106. DOI: 10.17352/2455-1759.000086The aim of this study was to evaluate the expression of Epidermal Growth Factor Receptor (EGFR) and Vascular Endothelial Growth Factor (VEGF) in patients with primary inverted papilloma (IP), recurrence of IP and carcinoma based on IP.
The study was performed on an archived material of the 52 patients that were surgically treated because of the primary IP, recurrent IP and carcinoma that developed on the ground of IP on the Tertiary care University Center in the period of 18 years. The expression of the EGFR and VEGF were measured by an immunohistochemistry method using commercially available antibodies.
There is a significant difference in expression of EGFR comparing primary IP to recurrence and squamous cell carcinoma (SCC) in the region of squamous and dysplastic epithelium, and also in the region of metaplastic epithelium between primary IP and carcinoma. There is a significant difference in expression of VEGF compared primary IP to recurrence and SCC in the region of the squamous and dysplastic epithelium.
The recurrence of IP presents step in tumour’s transformation between benign lesion and carcinoma.
Introduction
Inverted papilloma (IP) is a benign sinonasal epithelial tumour that belongs to a group of transitional cell papillomas [1,2]. It was first described by Vart, more than a hundred years ago, but the real nature of the disease was explained by Ringertz in 1938 [3].
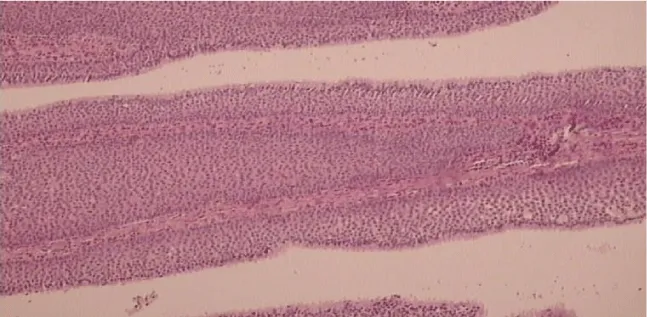
Inverted papilloma has a potential to infiltrate surrounding tissue, has a high recurrence rate and a potential of malignant transformation [4,5]. It grows on the mucosal surface in an exophytic form with a villous surface. Histologically the villi have a vascular and fibrous basis, covered by epithelium that invaginates into stroma at places, thus leading to its name: inverted papilloma4. The epithelium is usually squamous, but sometimes it can be cylindrical or mixed. Malignant alteration is morphologically seen as a cell polymorphism, the presence of pathological mitoses, desmoplastic stromal reaction, bone invasion, architectural disorder, paradoxical maturation, keratin pearl formation and high epithelial to stromal ratio [6,7].
Aetiology of the inverted papilloma remains unknown. Risk factors include outdoor and industrial factors; the role of human papilloma virus (HPV) is still controversial while inflammatory disease, smoking, and alcohol are not considered to be involved [8,9].
Dominant symptoms of inverted papillomas include unilateral nose congestion, which is often the only symptom, recurrent nasal bleeding, nasal discharge, sometimes blood tinged, and impaired olfactory function. Pain is sometimes present, but it is rarely the main symptom. Insufficient resection of the tumour, a large extension of the disease and the difficulty to reach surgically some of the locations where papillomas are situated, contribute to high recurrence rates. The other important factor is already multifocal nature of the tumour at the moment the diagnosis is set. In most of the patients recurrence is present 3 years after the initial operation, although recurrences 10 years postoperatively have been reported [9,10]. Therefore, all of the patients, including those without recurrent disease, demand long-term monitoring [11,12]. Malignant transformation is also well documented [13-16], and according to some studies, it takes about 63 months (6-153 months) for malignant transformation [17].
There are two options regarding the correlations between inverted papilloma and squamous cell carcinoma:
1. squamous cell carcinoma is present together with inverted papilloma at the time of diagnosis as a small focus or it forms the majority of the neoplasm (synchronous)
2. squamous cell carcinoma develops in the same anatomical unit where the inverted papilloma without malignant characteristics was previously resected (metachronous)
Because of this correlation, it is possible that inverted papilloma represents a form of the lesion with malignant transformation potential.
One of the important factors of malignant transformation is epidermal growth factor (EGF). It has an important role in the regulation of cellular growth and in their proliferation and differentiation [18]. It also marks increasing risk of the malignant transformation [14,19].
Its receptor – EGFR (EGFR) is a 170 kDa transmembrane receptor encoded by the human HER1 gene. The EGFR protein contains an extracellular ligand binding domain, a transmembrane region and an intracellular domain with intrinsic protein-tyrosine kinase activity. Ligand binding of the EGF receptor activates the EGFR tyrosine kinase which results in cell growth and differentiation [20]. It is more expressed in malignant cells and is related to poor prognosis and shorter survival of the patients, though it is present on the normal cells as well.
The other factor of the malignant transformation is vascular endothelial growth factor (VEGF). It is a signal protein and proangiogenic factor; it is secreted by tumoral and endothelial cells and has a very important role in tumour angiogenesis, growth and metastasis [21]. The whole VEGF family exerts their biological functions through the interaction with transmembrane receptors such as tyrosine kinase receptors VEGFR1 and predominantely VEGFR2. VEGFR2 stimulates cell proliferation and migration and thus promotes the formation of new vessels; which can produce plaque progression and eventusl hemorrhage [22-24].
Clinical and pathohistological analysis so far couldn't determine the characteristics of the tumour that will show its future biological development and clinical course in every single patient. High recurrence rates and the possibility of malignant transformation, together with the impossibility to predict in which cases will it occur, demands to find the objective characteristics, which will help to find adequate therapy and determine the need for close postoperative monitoring of every single patient. In that manner, the role of EGFR and VEGF is to be examinated.
The hypothesis in our study is that the patients with the recurrence of the inverted papilloma, or the carcinoma that developed on the ground of inverted papilloma, have higher expression of epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF).
Such finding could help to establish those patients who have a higher risk of recurrence or malignancy, and therefore require more careful monitoring and more aggressive treatment modalities.
Materials
With institutional review board approval, an archived material from 52 patients with inverted papilloma was obtained.
These patients were surgically treated at the tertiary care university hospital in the period from 1985 till 2003. 52 patients with primary tumour, recurrent tumour, and carcinoma that developed on the ground of IP were included in the study. They were both genders and none of them was previously treated with some other method.
The specimens were grouped into following three groups:
• Patients with inverted papilloma
• Patients with recurrent inverted papilloma
• Patients with carcinoma that developed on the ground of the previous IP
Reagents
The expression of the epidermal growth factor receptor and vascular endothelial growth factor were measured by immunohistochemistry method using commercially available antibodies. VEGF (C-1): sc-7269 (Santa Cruz Biotechnology, Inc.) and EGFR (Dako, Glostrup, Denmark) were used applying proposed manufacturer's protocol, and the PBS incubation was used as a negative control.
Methods
After the fixation in buffered formaldehyde and transfer into paraffin blocks, the tissue was cut to the thickness of 4-5 µm, and then put on the glass. After the fixation, the tissue was put to 70 0C during 2 hours. It was deparaffinised in xylene and rehydrated in ethanol solutions of decreasing concentrations. The activity of endogenic peroxidase was blocked with 3 % hydrogen peroxide solution during 10 minutes.
The standard avidin-biotin immunohistological method was used for dying using the automatic dyeing machine (TechMate, Dako) that uses capillary activity. At the end, specimens were dyed with haematoxylin-eosin.
Overnight incubation with VEGF antibodies was performed in 1% buffered phosphoric acid /bovine serum albumin (1% PBS-BSA) in 1:50 ratio. Optimal antibody concentration of 2,5 µg/ml, was determined by titration and was within recommended range (0,5-5 ug/ml). The specimens were washed three times with PBS for 2 minutes and incubated with biotin categorized anti- goat or mice IgG for 1 hour at the room temperature. Concentration of the antibody was also determined by titration, it was 3,5 µg/ml and was within recommended range of 1-5 µg/ml.
After washing 3 times with PBS during 2 minutes, the cuts were dyed with the streptavidin-peroxidase detection system. The incubation with PBS instead of antibody was used as a negative control.
Immunohistochemmistry
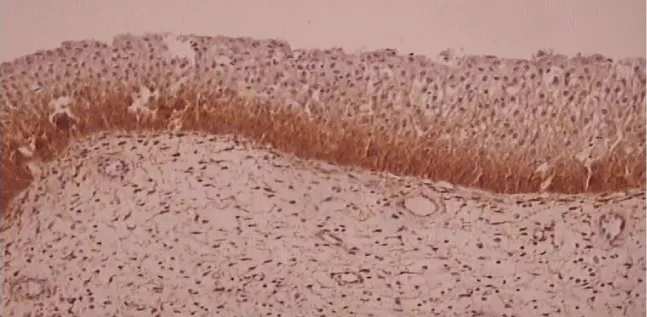
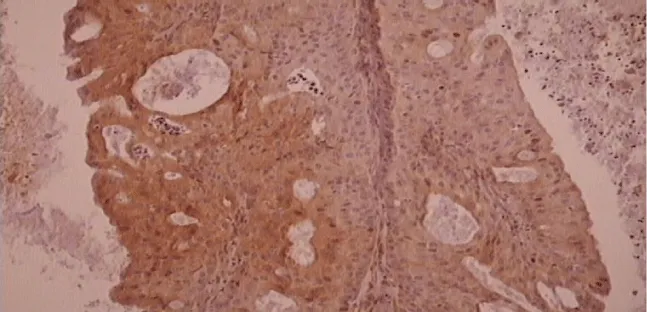
All specimens were examined by one pathologist in a semiquantitative manner [scoring 0-3. 0 – no reaction (up to 10% positive cells), 1 – weak reaction (up to 25% positive cells), 2 – middle reaction (26-50% positive cells), 3 – strong reaction (more than 50% positive cells)].
The intensity of the immunohistological reaction was determined in the tumour in 3 big magnification optical fields (magnification 40x) in the area of highest exposition ("hot spot") in following regions:
• cylindrical epithelium
• metaplastic epithelium
• squamous epithelium (more metaplastic)
• dysplastic epithelium
Statistical analysis
The results were analysed by non-parametric statistical tests. The differences between the groups for qualitative data will be analysed using Fisher exact test. Statistically significant were considered differences p<0.05.
Results
There were 52 patients included in the study, with the age between 18 and 72 years and the median of 54. They were divided into three groups - patients with primary IP (34 patients), patients with recurrent IP (11 patients) and patients with carcinoma on the ground of IP (7 patients).
EGFR expression was analysed in the area of cylindrical epithelium, that represents normal nasal mucosa, and in three zones of changed epithelium, metaplastic, dysplastic and squamous. There was no significant difference between groups in the area of cylindrical epithelium, i.e. the normal nasal mucosa. In the area of metaplastic epithelium, there was no significant difference between groups of patients with primary IP and recurrent IP, and recurrent IP and carcinoma, but the difference was significant between primary IP and carcinoma groups.
In the area of squamous epithelium, that represents the area of stronger metaplastic transformation, there was significant difference between groups of patients with primary IP and recurrent IP, and primary IP and carcinoma while the difference between groups of patients with recurrent IP and carcinoma was not significantly different.
In the area of dysplastic epithelium, that represents the heaviest transformation, the relations are similar: significant between groups with primary and recurrent IP, and groups with primary IP and carcinoma, while the difference was not significant between groups with recurrent IP and carcinoma.
Similar results are obtained in the squamous epithelium area regarding the expression of VEGF between patients with primary IP and recurrent IP, and the groups of patients with primary IP and carcinoma, while there was no significant difference between recurrent IP patients and the ones with the carcinoma.
The results in the dysplastic epithelium areas were also similar.
Results of the immunohistochemical analysis on EGFR and VEGF are shown in the tables below (Tables 1-4). Figures of inverted papilloma and EGFR and VEGF expression are represented in figures 1-3.
Discussion
Inverted papilloma is a rare sinonasal tumour and represents 0,5 – 7% of all tumours in the sinonasal region [1,2]. It has a high recurrence rate and a high malignant potential. It is characterized by stromal invasion, thus if the resection of the inverted papilloma is insufficient, the recurrence rate is high [3]. It amounts, according to different studies, between 5.7–32% [25]. On the other side, the correlation among inverted papilloma and squamous cell carcinomas is well documented. Current literature shows this correlation somewhere between 10-25 % [13-16].
Measuring of the expression of EGFR and VEGF could help to determine the patients with high risk of recurrence and carcinoma.
There were 52 patients, 37 males and 15 females included in the study. The number of male patients was 2.5 times higher, which supports the data from the literature about the higher incidence of inverted papilloma in males [3,26]. There was a significant difference in expression of EGFR in metaplastic epithelium between primary IP and carcinoma groups. In groups of squamous and dysplastic epithelium significant were differences between of patients with primary and recurrent IP, and primary IP and carcinoma.
In the study of Katori et al. [19], significant increase of EGFR was observed in IP with severe dysplasia, IP with carcinoma and invasive carcinoma compared to IP with mild dysplasia and control group. According to the study of Kong et al. [27], there was a significant difference between EGFR expression in severe atypical hyperplasia of IP then that in mild atypical hyperplasia of IP, i.e. those first was much stronger. That means the stronger the epithelium transformation is, the stronger expression of EGFR is.
In the study of Chao JC et al. [28], investigated was the expression of EGFR in IP and synchronous carcinoma and in IP and metachronous carcinoma. This expression was much stronger when compared to normal mucosa and polypoid nasal mucosa, which suggested a possible role of EGFR in the malignant transformation of IP. The study of Sauter A. et al. [2], also confirmed that an increased EGFR expression is associated with early events in IP carcinogenesis.
In VEGF expression, in the region of squamous and dysplastic epithelium, there was the statistically significant difference between patients with primary and recurrent IP and primary IP and carcinoma.
In the study of Kong et al. [27], expression of VEGF and his receptor in epithelium was significantly stronger in severe atypical hyperplasia than that in mild atypical hyperplasia and was significantly stronger in mild atypical hyperplasia than in inverted papilloma.
There are also studies that showed that expression of VEGF and his receptor is also stronger expressed in the tissue of inverted papilloma than in normal sinus nasal mucosa i.e. inferior turbinates [30,31]. Those studies have shown that the expression of EGFR and VEGF and his receptor is the stronger, the more epithelium changed is. Also, EGFR could have a role in the process of malignant transformation. This study has supported the data from the literature.
Thus the findings of EGFR and VEGF proteins could help to determine the group of patients with inverted papilloma with the higher risk of recurrence or malignant transformation, and to determine the need for more intensive monitoring of those patients.
Also, different ways to determine EGFR and VEGF could be used, such as RT-PCR, which would enable the real quantification of the results.
Another limitation of the study is its retrospective nature and small sample which are result of the fact that the disease is still pretty rare. The lack of control group is due the fact that there are known expressions of these antibodies in healthy tissues in every chart that comes with antibody. Anyway, multicenter study with a large series of cases should be performed to confirm our results.
Future investigations are also still needed. Beside the fact that all patients with inverted papilloma should be monitored more closely, especially in recurrent papillomas, it is also possible to reveal and emphasise the importance of the development of targeted therapy such as monoclonal antibodies against tumour cells. This treatment would give a totally new dimension in the treatment of patients with recurrent inverted papilloma or carcinoma, and it would open them new possibilities.
Ethical statement
The study was approved by institutional review board of the University center. All known ethical principles were respected.
- Yu HX, Liu G (2014) Malignant transformation of sinonasal inverted papilloma: A retrospective analysis of 32 cases. OncolLett 8: 2637–2641. Link: https://goo.gl/XfWRSd
- Sauter A1, Matharu R, Hörmann K, Naim R (2007) Current advances in the basic research and clinical management of sinonasal inverted papilloma (review). Oncol Rep 17: 495–504. Link: https://goo.gl/2hvjGZ
- Ringertz N (1938) Pathology of malignant tumors arising the nasal and paranasal cavities and maxilla. ActaOtolaryngolSuppl (Stock) 27: 31-42. Link: https://goo.gl/9WvYiv
- Bielamowicz S, Calcaterra TC, Watson D (1993) Inverting papilloma of the head and neck: The UCLA update. Otolaryngol Head Neck Surg 109: 71-76. Link: https://goo.gl/btgGFT
- Michaels L, Young M (1995) Histogenesis of papillomas of the nose and paranasal sinuses. Arch Pathol Lab Med 119: 821-826. Link: https://goo.gl/j6GZeu
- Nudell J, Chiosea S, Thompson LD (2014) Carcinoma Ex-Schneiderian Papilloma (Malignant Transformation): A Clinicopathologic and Immunophenotypic Study of 20 Cases Combined with a Comprehensive Review of the Literature. Head Neck Pathol 8: 269–286. Link: https://goo.gl/r7jtUa
- Eggers G1, Eggers H, Sander N, Kössling F, Chilla R (2005) Histological features and malignant transformation of inverted papilloma. Eur Arch Otorhinolaryngol 262: 263–268. Link: https://goo.gl/5gWuv8
- Lin GC, Scheel A, Akkina S, Chinn S, Graham M, et al. (2013) P16, EGFR, Cyclin D1, and p53 Staining Patterns for Inverted Papilloma. Int Forum Allergy Rhinol 3: 885–889. Link: https://goo.gl/xjwdc4
- Vorasubin N, Vira D, Suh JD, Bhuta S, Wang MB, et al. (2013) Schneiderian papillomas: Comparative review of exophytic, oncocytic, and inverted types. Am J Rhinol Allergy 27: 287–292. Link: https://goo.gl/nCWsBq
- Mirza S1, Bradley PJ, Acharya A, Stacey M, Jones NS (2007) Sinonasal inverted papillomas: Recurrence, and synchronous and metachronous malignancy. J Laryngol Otol 121: 857–864. Link: https://goo.gl/RxkqbS
- Nielsen PL1, Buchwald C, Nielsen LH, Tos M (1991) Inverted papilloma of the nasal cavity: pathological aspects in a follow-up study. Laryngoscope 101: 1094-1101. Link: https://goo.gl/2F7VUx
- Klimek T1, Atai E, Schubert M, Glanz H (2000) Inverted papilloma of the nasal cavity and paranasal sinuses: clinical data, surgical strategy and reccurence rates. Acta Otolaryngol 120: 267-272. Link: https://goo.gl/qjjfyg
- But-Hadzic J, Jenko K, Poljak M, Kocjan BJ, Gale N, et al. (2011) Sinonasal inverted papilloma associated with squamous cell carcinoma. Radiol Oncol 45: 267–272. Link: https://goo.gl/Y3V6Js
- Udager AM, Rolland DCM, McHugh JB, Betz BL, Murga-Zamalloa C, et al. (2015) High-Frequency Targetable EGFR Mutations in Sinonasal Squamous Cell Carcinomas Arising from Inverted Sinonasal Papilloma. Cancer Res 75: 2600-2606. Link: https://goo.gl/LfcjU9
- Sulica RL1, Wenig BM, Debo RF, Sessions RB (1999) Schneiderian papillomas of the pharynx. Ann Otol Rhinol Laryngol 108: 392-397. Link: https://goo.gl/a2ULCp
- Tsue TT1, Bailet JW, Barlow DW, Makielski KH (1997) Bilateral sinusal papilloma in aplastic maxillary sinuses. Am J Otolaryngol 18: 263-268. Link: https://goo.gl/tAWYTt
- Liang QZ, Li DZ, Wang XL, Huang H, Xu ZG, et al. (2015) Survival Outcome of Squamous Cell Carcinoma Arising from Sinonasal Inverted Papilloma. Chin Med J 128: 2457-2461. Link: https://goo.gl/sLHXF8
- Shimizu N1, Wang Y, Minoshima S, Ishitoya J (1994) Detection of epidermal growth factor receptor gene amplification in human squamous cell carcinoma using fluorescence in situ hybridization. Jpn J Cancer Res 85: 567-561. Link: https://goo.gl/ip9uRW
- Katori H, Nozawa A, Tsukuda M (205) Markers of malignant transformation of sinonasal inverted papilloma. Eur J Surg Oncol 31: 905-911. Link: https://goo.gl/kkxPi9
- Sliwkowski MX1, Lofgren JA, Lewis GD, Hotaling TE, Fendly BM, et al. (1999) Nonclinical studies addressing the mechanism of action of Trastuzumab (Herceptin). Semin Oncol 26 (suppl 12): 60-70. Link: https://goo.gl/NpGFNr
- Ferrara N (2002) VEGF and the quest for tumor angiogenesis factors. Nat Rev Cancer 2: 795-803. Link: https://goo.gl/vMshhQ
- Farzaneh Behelgardi M, Zahri S, Mashayekhi F, Mansouri K, Asghari SM (2018) A peptide mimicking the binding sites of VEGF-A and VEGF-B inhibits VEGFR-1/-2 driven angiogenesis, tumor growth and metastasis. Sci Rep 8: 17924. Link: https://goo.gl/NeZLUi
- Yang Z1, Wang H1, Jiang Y1, Hartnett ME (2014) VEGFA activates erythropoietin receptor and enhances VEGFR2-mediated pathological angiogenesis. Am J Pathol 184: 1230–1239. Link: https://goo.gl/XtQW4y
- Yuan R, Shi W, Xin Q, Yang B, Hoi MP, et al.(2018) Tetramethylpyrazine and Paeoniflorin Inhibit Oxidized LDL-Induced Angiogenesis in Human Umbilical Vein Endothelial Cells via VEGF and Notch Pathways. Evid Based Complement Alternat Med 2018: 3082507. Link: https://goo.gl/eBvLCk
- Lombardi D1, Tomenzoli D, Buttà L, Bizzoni A, Farina D, et al. (2011) Limitations and complications of endoscopic surgery for treatment for sinonasal inverted papilloma: a reassessment after 212 cases. Head Neck 33: 1154–1161. Link: https://goo.gl/g3JRkR
- Díaz Molina JP1, Llorente Pendas JL, Rodrigo Tapia JP, Alvarez Marcos C, Obeso Agüera S, et al. (2009) [Inverted sinonasal papillomas. Review of 61 cases]. Acta Otorrinolaringol Esp 60: 402-408. Link: https://goo.gl/oe4Vhv
- Kong H1, Sun X, Wu X, Guan Q, Zou C. (2005) The significance and expression of EGF and its receptor in nasal inverted papillomas and the correlation with malignant phenotype. Lin Chuang Er Bi Yan Hou Ke Za Zhi 19: 193-4, 197. Link: https://goo.gl/dXFXha
- Chao JC, Fang SY (2008) Expression of epidermal growth factor receptor in the inverted papilloma and squamous cell carcinoma of nasal cavity. Eur Arch Otorhinolaryngol 265: 917-922. Link: https://goo.gl/JxKqh3
- Kong H1, Guan Q, Sun X, Wang N (2012) Significance and expression of VEGF and its receptor in nasal inverted papillomas and the correlation with malignant phenotype. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 26: 337-8, 342. Link: https://goo.gl/WztkrY
- Han FJ1, Wang YP, Yang JH, Guan GM, Dong Z, et al. (2000) The significance and expression of vascular endothelial growth factor on nasal inverted papillomas. Lin Chuang Er Bi Yan Hou Ke Za Zhi 14: 65-67. Link: https://goo.gl/SibsmZ
- Liu W1, Li Z, Luo Q, Lai Y, Zhang J, et al. (2011) The elevated expression of osteopontin and vascular endothelial growth factor in sinonasal inverted papilloma and its relationship with clinical severity. Am J Rhinol Allergy 25: 313-317. Link: https://goo.gl/1AKKjr
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
