Archives of Otolaryngology and Rhinology
Diffusion-weighted magnetic resonance imaging with echo-planar and non-echo-planar (PROPELLER) techniques in the clinical evaluation of cholesteatoma
María Dolores Moreno-Ramos1*, Miguel Olivencia Pérez2, Juan Antonio Ibáñez Rodríguez2, Mª José Gómez Galán2 and Francisco Javier Ramos Medrano1
2Department of Otorhinolaryngology. Hospital San Juan de Dios del Aljarafe. Avda San Juan de Dios s/n, 41830-Bormujos, Seville, Spain
Cite this as
Moreno-Ramos MD, Pérez MO, Ibáñez Rodríguez JA, Gómez Galán MJ, Ramos Medrano FJ (2019) Diffusion-weighted magnetic resonance imaging with echo-planar and non-echo-planar (PROPELLER) techniques in the clinical evaluation of cholesteatoma. Arch Otolaryngol Rhinol 5(1): 014-019. DOI: 10.17352/2455-1759.000089Background: Diffusion-weighted magnetic resonance imaging (DWI) is an alternative to second-look surgery for the detection of cholesteatoma.
Purpose: To assess the utility of DWI with echo-planar (EPI-DWI) and non-echo-planar (PROPELLER) sequences for the diagnosis of primary and recurrent cholesteatoma.
Materials and methods: A prospective study of 33 ears, 21 with previous cholesteatoma surgery. Twelve patients were asymptomatic, with 4 showing signs suggestive of cholesteatoma in previous CT scans. The MRI protocol was: axial and coronal T1-weighted and T2-weighted imaging, and diffusion-weighted sequences by both EPI-DWI and PROPELLER techniques. The results were correlated with the clinical examination and subsequent surgical findings. Ten patients undergoing ear surgery for other reasons were included as negative controls.
Results: The diagnostic accuracy was calculated with the 22 patients who underwent surgery and the negative controls. Both diffusion-weighted sequences showed a specificity of 100%. The sensitivity of PROPELLER was 95%, compared to 20% for EPI-DWI. The latter showed non-specific imaging with bone artefacts, thus making impossible to confirm or exclude the diagnosis. The PROPELLER technique yielded one false negative, compared with 16 by EPI-DWI. Both techniques gave a false negative in one case of a primary cholesteatoma. A positive result was obtained in two patients with no clinical suspicion of recurrence.
Conclusions: In contrast to EPI-DWI, PROPELLER is a reliable technique for diagnosing cholesteatoma. As positive results were found in asymptomatic patients, we recommend regular monitoring by PROPELLER, even in the absence of clinical findings.
Introduction
A cholesteatoma is a benign, pseudotumoral lesion formed by accumulation of keratinized squamous epithelium within the cavities of the middle ear. It can behave aggressively because of ability to destroy bone and affect the facial nerve and inner ear, and can even cause intracranial complications. As such, the treatment of choice is surgery. It can be congenital or acquired, with the latter being more common, especially in chronic otitis media with marginal perforations of the tympanic membrane. Although the diagnosis is essentially clinical (by direct examination), this is usually accompanied by imaging techniques, especially in patients with suspected recurrence, as an alternative to second-look surgery.
In light of problems posed by computed tomography (CT) [1,2], magnetic resonance imaging (MRI) has been considered to be a viable option given its greater ability to distinguish between different tissues [3]. Studies in this regard have been conducted with intravenous contrast followed by T1-weighted image acquisition 40 to 60 minutes after contrast administration [4,5]. Diagnosis is based on an absence of contrast uptake. However, interpretation of the results requires expertise [6]. Diffusion-weighted imaging (DWI) based on restricted water movement [7], has been proposed as an alternative [3,8-17], because of the high keratin content. Thus, whereas, echo-planar imaging (EPI-DWI) has the disadvantages of lower spatial resolution and the presence of artefacts due to the proximity of bone [8,18], the introduction of non-echo-planar imaging (non-EPI-DWI) has largely minimised the second issue, and improved resolution for detecting smaller lesions [8,9,12].
The aim of this study was to assess the utility of EPI-DWI and non-EPI (periodically rotated overlapping parallel lines with enhanced reconstruction- PROPELLER-DWI) diffusion-weighted MRI, for the diagnosis of both primary and recurrent cholesteatoma, and to correlate the results with clinical and surgical findings. We want to determine which DWI-technique should be used, if it would be advisable to use them to monitor asymptomatic patients and also establish if there are appropriate to diagnose primary cholesteatoma.
Materials and methods
This was a prospective study of 30 consecutive patients, six of whom had bilateral involvement. Three patients with unilateral involvement did not attend their appointment after the MRI and were excluded. A total of 33 ears were therefore examined.
A total of 21 of the ears examined had a history of previous cholesteatoma surgery. Recurrence was suspected in 8 of these, and a further 8 presented no clinical manifestations or examination findings (one of these ears belonged to a patient with clinical manifestations in the other ear). One ear could not be assessed by examination because of postsurgical stenosis of the external auditory canal. The diagnosis was uncertain for 2 ears, and in a further 2 a previous CT scan suggested recurrence even though there was no clinical evidence of this. There was no history of surgery in 12 cases: 10 of these had suggestive clinical findings, and, in the remaining 2, a previous CT scan was suggestive but, as with the other group, there was no evidence on examination. Ten patients who underwent ear surgery for other reasons were included as negative controls prior to surgery. None of them showed signs of cholesteatoma during the intervention nor showed a positive result by DWI. Table 1 summarizes the patients included in the study.
Twenty two patients underwent surgery. The diagnostic accuracy was calculated with the 22 operated patients and the 10 negative controls. The gold standard for all of them was the surgery. The other 11 patients were not operated and they were not taken into account for calculating diagnostic accuracy. But they have been clinically followed up with no suspicion of recurrence to date.
MRI was performed using a 1.5 T unit (Signa. General Electric Medical Systems, USA), with an 8-channel head coil (8 NV array) with sequences in the axial plane: T1-weighted spin echo (TR 340/TE 14, matrix 256x224 and NEX 3), T2-weighted axial fast spin echo (TR 4200/TE 89.8, matrix 384x256 and NEX 4), PROPELLER diffusion-weighted imaging (FSE TR 5200/TE 97.7, matrix 128x128, NEX 1.5, b factor value 1000 and 800) and echo-planar diffusion-weighted imaging (SE/EPI TR 8800/TE 95.3, matrix 132x132, NEX 2 and b factor value 1000). These sequences were synchronised using the same FOV (22 cm) and the same spatial slice programming. Slices had a thickness of 3 mm, with no intersection gap. This technical detail is very important for subsequent image evaluation. The sequences in the coronal plane were as follows: T2-weighted fast spin echo (TR 6900/TE 89.2, matrix 384x256 and NEX 4, FOV 18 cm, thickness 2 mm and intersection gap 0.5 mm) and T1-weighted spin echo (TR 500/TE 14, matrix 256x224 and NEX 3, FOV 18 cm, thickness 2 mm and intersection gap 0.5 mm). The diffusion-weighted images obtained were then subjected to post-processing at the work station in order to obtain apparent diffusion coefficients (ADC) maps. Unenhanced CT scans (Brilliance 16-slice, Phillips, Holland) were obtained for the temporal bone, with a slice thickness of 0.65 mm, an intersection gap of 0.3 mm, and separate post-processing for each ear, for subsequent multiplanar reconstruction. CT was used to establish anatomical correlations and assess bone erosion. All imaging (CT and MRI) was interpreted by the same radiologist, who was blinded to the clinical history and examination, in separate sessions for the DWI-sequences. The diagnosis of cholesteatoma on DWI was based on the presence of increased signal intensity on b value 0 s/mm2 that persists or increases on b value 800/1000 s/mm2, decreased signal intensity on ADC map and a soft-tissue mass hyperintense on T2-weighted images and hypointense on T1-weighted images. We use these two sequences for better localisation of the disease. The ADC values have not been used as a diagnosis criterion since no clear values have yet been establish [6].
Medical records were reviewed, and imaging data were correlated with surgical findings in patients who underwent surgery following radiological examination. Descriptive statistics were calculated for the variables patient age (mean and standard deviation) and sex (total numbers and percentages). Sensitivity and specificity results and positive and negative predictive values were obtained for the PROPELLER-DWI and EPI-DWI techniques in comparison with surgical findings, and 95% confidence intervals (95%CI) were calculated. Statistical analysis was performed following the recommendations published by Rodríguez Artalejo et al. [19].
Results
The mean patient age was 48.66 years (range of 17 to 75 years and standard deviation: 14.45), with 60% of patients being male and 40% female.
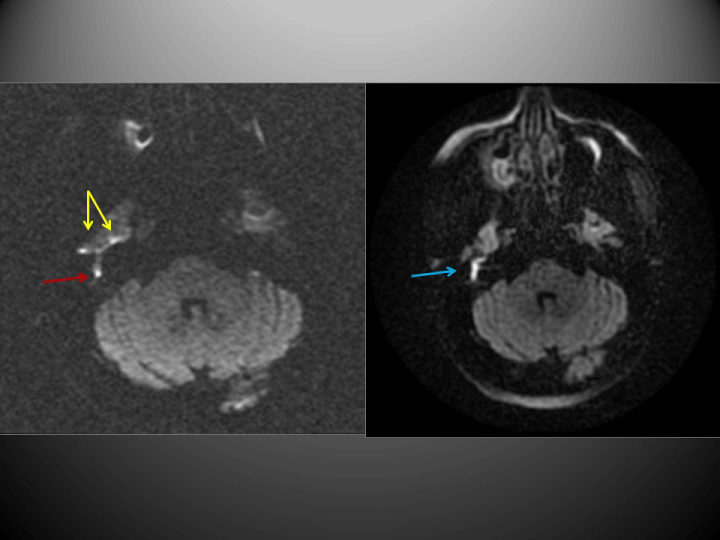
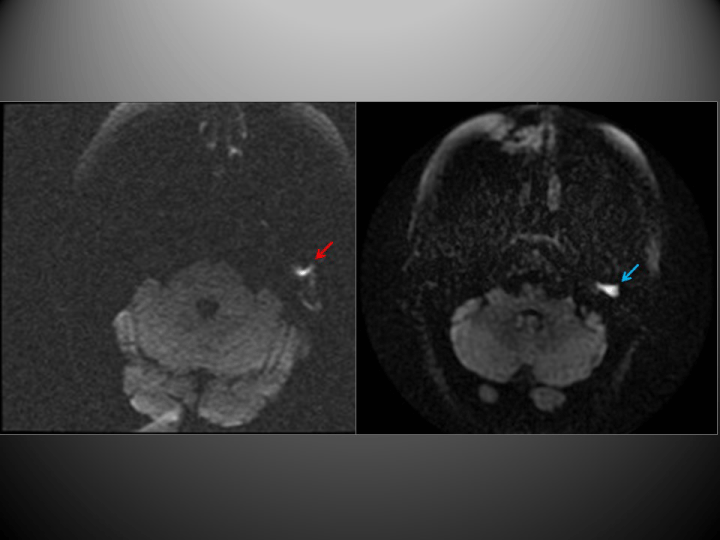
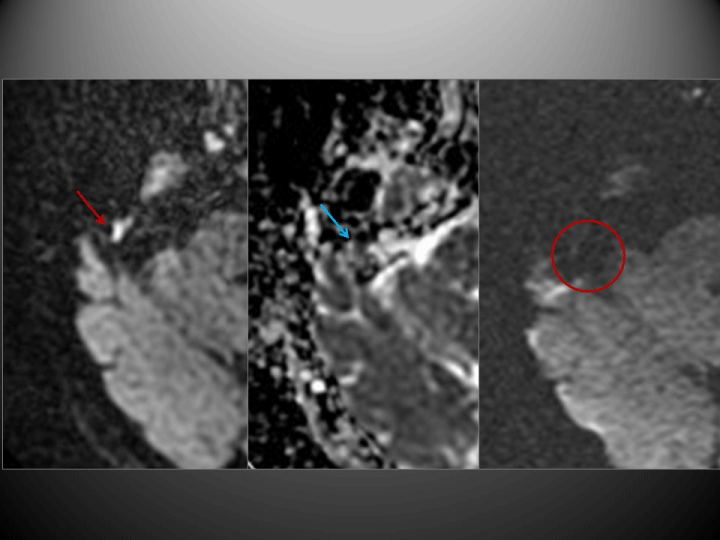
The PROPELLER-DWI technique yielded findings consistent with cholesteatoma in 19 cases. EPI-DWI was also positive in four of these cases, although this technique underestimated lesion size and artefacts due to bone proximity interfered with the images obtained (Figure 1). Moreover, on another 6 occasions this technique produced findings that were not sufficiently specific to allow a diagnosis to be made (Figure 2). All other cases were negative (Figure 3), as were the negative controls.
Due to their clinical relevance and management, the following two cases, both of which were diagnosed using PROPELLER-DWI, are highlighted:
In one case in which CT showed erosion of the tegmentum tympani, MRI proved conclusive for assessing temporal lobe herniation, which is essential when planning surgery.
One case of atypical location, with occupation of the epitympanum and no perforation of the tympanic membrane, was found in an adult patient.
None of the cases (n=4) with a suggestive CT scan proved positive by either DWI technique. No differences were found when the PROPELLER-DWI sequence was performed with b factor values of 1000 and 800.
A total of 22 patients underwent surgery, including all 19 whose PROPELLER-DWI scans were positive, and three whose results by this technique were negative. Surgical findings in these patients were correlated with the MRI results obtained (Tables 2,3). In the group that had undergone surgery previously (n=12), a cholesteatoma was found during the intervention in all patients whose PROPELLER-DWI scan was positive. This included two patients in whom no clinical suspicion existed. Only two cases were diagnosed correctly by EPI-DWI. In one case in which the CT scan suggested cholesteatoma, nothing was found during the intervention, thus confirming the MRI results (Table 2). In the “no previous surgery” group (n=10), the aetiology of the condition was confirmed in the 8 cases that proved positive by the PROPELLER-DWI technique. As in the first group, only two patients were correctly diagnosed by EPI-DWI. One patient with negative results by both DWI techniques yielded no findings on surgery. One false negative result was obtained by both techniques (Table 3).
Sensitivity and specificity values were calculated for each diffusion-weighted sequence using the data obtained from patients with histological confirmation plus negative controls (Tables 4,5). The results for the PROPELLER-DWI technique were: sensitivity 95% (76.4–99.1%), specificity 100% (75.7–100%), positive predictive value (PPV) 100% (83.2–100%) and negative predictive value (NPV) 92.3% (66.7–98.6%). The corresponding values for the EPI-DWI technique were: sensitivity 20% (8.1–41.6%), specificity 100% (75.7–100%), PPV 100% (51.0–60.9%) and NPV 42.9% (26.5–24.3%).
Discussion
Non-EPI-DWI techniques represent a major advance in the diagnosis of cholesteatoma, especially for detecting recurrence. The high sensitivity and specificity values for PROPELLER obtained in our study agree with most published findings [3,6,8,17,]. In contrast, Kasbekar et al. [9], obtained low sensitivity (29%), and concluded that the technique cannot detect cholesteatomas smaller than 4 mm. These authors explained their findings by the fact that they used a 1.5 T MRI scanner. The matrix used (128x128) might have resulted in decreased sensitivity for small lesions [6]. In our series, however, using a 1.5 T scanner and the same matrix, we were able to diagnose 3 mm lesions (range: 3-22 mm), as was also the case for other authors using a 256x256 matrix [3,6]. In any case, from a clinical point of view, it is widely accepted that under-diagnosed lesions of less than 2‑3 mm are not clinically relevant and may be eligible for periodic monitoring to detect any growth [20].
The utility of non-EPI-DWI techniques is supported by numerous studies, most of which used the half-Fourier acquisition single-shot turbo spin-echo (HASTE) technique [10,11,15]. In contrast, there are few published studies based on PROPELLER-DWI, which is the technique used at our institution. This is a multi-shot fast spin echo sequence with radial data acquisition in the k-space. It provides fast imaging and good resolution, with less distortion and fewer artefacts in scans of the skull base when compared with EPI-DWI sequences [8]. In contrast to HASTE, in which the plane can be coronal or axial, in this technique, sequences can only be acquired in the axial plane. HASTE requires separate acquisitions for each b value and the measurement parameters and slice positions for both to be identical. As a result, this technique is more susceptible to both motion artifacts and to slice position misregistration errors in post-acquisition ADC map calculations. However, PROPELLER can acquire two b values in a single scan, thus reducing the acquisition time and therefore removing the problems inherent to the HASTE sequence [21]. Studies comparing these two techniques have not yet been performed [6]. Although a new EPI-DWI sequence (RESOLVE) introduced recently has provided promising results, it is not available at all institutions and there are no comparative studies with non-EPI-DWI sequences [22,23].
From a technical point of view, it should be noted that it is very important to synchronise PROPELLER-DWI studies in the axial plane with the basic sequences in order to be able to evaluate images more efficiently. Bearing this issue in mind, interpretation is more straightforward than with EPI-DWI, although there is a learning curve. In one case in our series, the technique was able to detect multiple foci. A description of these sites provided guidance for the surgeon, thereby allowing proper surgical planning [11,17].
In agreement with previously published studies [8,18,24,25], we found EPI-DWI difficult to evaluate as this technique is markedly influenced by geometric distortion artefacts in air-bone interface regions at the skull base, thus making impossible to confirm or exclude the suspected diagnosis. The sensitivity and NPV of this technique were therefore low (20% and 42.9% respectively). Our sensitivity and NPV data are lower than published results, which are inconsistent, ranging from 76% to 29% for sensitivity and 66% to 33% for NPV [8,9,13,26].
False positives are usually due to recent surgery with residual haemorrhage, cholesterol granulomas [1] and abscesses [1,3], as well as reconstruction with bone powder [18], although they tend to be easy to identify based on clinical findings [6]. There were no false positives in our series. Like other authors [3,11,14,16,27,28], we obtained some false negatives: 16 by EPI-DWI and only one by PROPELLER-DWI. The latter was a false negative by both techniques and its size on surgery was 5 mm. The presence of retraction pockets and mural cholesteatomas with little keratin content limits the utility of diffusion-weighted MRI as they produce no hyperintensity [3]. Their size also has an influence as they below the technique’s limit of detection when smaller than 3 mm by non-EPI-DWI [1]. In contrast, with EPI-DWI techniques, the sensivity reaches 100% only when the size of the lesion is equal or greater than 5 mm. The lowest size of cholesteatoma detected seems to range from 4 to 5 mm [29,30,31]. Venail et al [29], only reported the diagnosis of 1 lesion of 3 mm. And in the study by Aikele et al [30], the three missed lesions were smaller than 5 mm. These data confirm the superiority of non-EPI-DWI techniques face to EPI-DWI. Motion artefacts are another limitation described in studies using the non-echo-planar diffusion-weighted HASTE technique [11,12]. We believe the high number of false negatives obtained by EPI-DWI to be a consequence of this technique’s limitations, rather than being due to the above reasons. Although the actual number of false-negatives may be underestimated as the majority of these patients were not operated upon, all patients have been clinically followed up with no suspicion of recurrence to date. In the case of the false negative obtained by the PROPELLER-DWI technique, we have found no plausible explanation for this misdiagnosis as, even upon review of the images, there is still no hyperintensity. A literature review found another similar case [32], in which the suggested explanation was that keratin content may vary depending on the age of the cholesteatoma. Given the possibility of false negatives, we agree that periodic clinical monitoring should be carried out even if the scan is negative [14,33], and the examination should be combined with basic T1- and T2-weighted sequences in order to avoid errors, for example from interposed fat from a previous intervention [34].
The fact that the misdiagnosis occurred in the group of patients who had not undergone surgery previously raises the question of whether the technique is less reliable in patients with a primary cholesteatoma. Published results in this regard are inconsistent, irrespective of the DWI technique used. Thus, whereas Profant et al. [13], only encountered misdiagnoses in the primary cholesteatoma patient group, Kasbekar et al. [9], only detected them in patients with a recurrence. On the other hand, Garrido et al. [16] and Evlice et al. [26], reported misdiagnoses in both groups. Although these misdiagnoses were more numerous in the primary cholesteatoma group, the differences were not statistically significant. Lastly, Pizzini et al. [15], obtained 100% sensitivity and specificity in both groups, as was also the case in a recent meta-analysis [33], which showed comparable results for primary and post-surgical groups. As our case series is small, we cannot draw any conclusions regarding this aspect.
We emphasise that non-EPI-DWI proved positive in two patients with no clinical signs, and these findings were confirmed during surgery. We have not found any similar references in the literature. These results suggest that some cases might be inaccessible to the clinician by otoscopy as a result of their location or small size. Alternatively, examination may not be feasible, for example because of a narrow external auditory canal, as was the case with one of our patients. As a result, we suggest monitoring these patients periodically by MRI even if they are asymptomatic.
CT has traditionally been regarded as the radiological technique of choice to accompany a diagnosis of primary and recurrent cholesteatoma. However, this technique has low specificity for distinguishing cholesteatoma from other tissues (fibrosis, granulation tissue, inflammation), especially in patients who have already undergone surgery and in whom recurrence is suspected, as well as in cases of atypical location or dubious diagnosis [2,35], leading to unnecessary surgery. Our findings support these findings as none of the patients with a suggestive CT scan yielded a positive result by DWI, and in the case in which surgery was performed, no cholesteatoma was seen during the intervention. CT should be omitted in cases that prove negative by PROPELLER-DWI, thereby reducing the radiation dose in such patients, who are usually monitored by this technique.
There are some limitations in this study. The number of cases is low and, as we have described before, the number of false-negatives may be underestimated as 11 patients were not operated upon. Moreover, the images interpretation was done by a single radiologist, therefore we were unable to establish interobserver agreement.
In conclusion, non-EPI-DWI by PROPELLER-DWI is a reliable technique for diagnosing both recurrent and primary cholesteatoma, thus greatly assisting decision-making by the otorhinolaryngologist. With EPI-DWI, in contrast, interpretation is hindered by the presence of artefacts. As we found positive results in asymptomatic patients, we recommend regular monitoring by PROPELLER-DWI, even in the absence of clinical findings.In conclusion, non-EPI-DWI by PROPELLER-DWI is a reliable technique for diagnosing both recurrent and primary cholesteatoma, thus greatly assisting decision-making by the otorhinolaryngologist. With EPI-DWI, in contrast, interpretation is hindered by the presence of artefacts. As we found positive results in asymptomatic patients, we recommend regular monitoring by PROPELLER-DWI, even in the absence of clinical findings.
- Li P, Linos E, Gurgel RK (2012) Evaluating the Utility of Non–Echo-Planar Diffusion-Weighted Imaging in the Preoperative Evaluation of Cholesteatoma: A 1-analysis. Larincoscope 28: 1-4.
- Cimsit NC, Cimsit C, Baysal B, Ruhi IC, Ozbilgen S, et al. (2010) Diffusion-weighted MR imaging in postoperative follow-up: reliability for detection of recurrent cholesteatoma. Eur J Radiol 74: 121-123. Link: https://goo.gl/cx2kJT
- Mateos-Fernández M, Mas-Estellés F, Paula-Vernetta C (2012) Papel de la resonancia magnética de difusión en el diagnóstico y seguimiento del colesteatoma. Estudio con la técnica PROPELLER difusión. Acta Otorrinolaringol Esp 63: 436-442. Link: https://goo.gl/DTe1Vz
- Williams MT, Ayache D, Alberti C (2003) Detection of postoperative residual cholesteatoma with delayed contrast-enhanced MR imaging: initial findings. Eur Radiol 13: 169-174. Link: https://goo.gl/BZMtYj
- Ayache D, Williams MT, Lejeune D (2005) Usefulness of delayed postcontrast magnetic resonance imaging in the detection of residual cholesteatoma after canal wall-up tympanoplasty. Laryngoscope 115: 607-610. Link: https://goo.gl/BiFAPi
- Más-Estellés F, Mateos-Fernández M, Carrascosa-Bisquert B (2012) Contemporary Non–Echo-planar Diffusion-weighted Imaging of Middle Ear Cholesteatomas. RadioGraphics 32: 1197-1213. Link: https://goo.gl/VY1emj
- Dietrich O, Biffar A, Baur-Melnyk A (2010) Technical aspects of MR diffusion imaging of the body. Eur J Radiol 76: 314-322. Link: https://goo.gl/EGvkAV
- Lehmann P, Saliou G, Brochart C (2009) 3T MR imaging of postoperative recurrent middle ear cholesteatomas: value of periodically rotated overlapping parallel lines with enhanced reconstruction diffusion-weighted MR imaging. AJNR 30: 423-427. Link: https://goo.gl/49Tmi9
- Kasbekar AV, Scoffings DJ, Kenway B (2011) Non echo planar, difussion-weigthed magnetic resonance imaging (periodically rotated overlapping parallel lines with enhanced reconstruction sequence) compared with echo planar imaging for the detection of middle-ear cholesteatoma. J Laringol Otol 125: 376-380. Link: https://goo.gl/K2G3Qr
- Alzérreca E, Garrido C, Zamorano R (2011) Resonancia magnética cerebral con secuencia difusión – HASTE en la evaluación clínica del colesteatoma. Rev. Otorrinolaringol. Cir. Cabeza Cuello 71: 249-256. Link: https://goo.gl/UQ1Fpb
- Turan Ilıca A, Hıdır Y, Bulakbaşı N (2012) HASTE diffusion-weighted MRI for the reliable detection of cholesteatoma. Diagn Interv Radiol 18: 153-158. Link: https://goo.gl/GHxfs9
- De Foer B, Vercruysse JP, Bernaerts A (2008) Detection of postoperative residual cholesteatoma with non-echo-planar diffusion-weighted magnetic resonance imaging. Otol Neurotol 29: 513-517. Link: https://goo.gl/cxrUXs
- Profant M, Sláviková K, Kabátová Z (2012) Predictive validity of MRI in detecting and following cholesteatoma. Eur Arch Otorhinolaryngol 269: 757-765. Link: https://goo.gl/T2GG3x
- Velthuis S, van Everdingen KJ, Quak JJ (2014) The value of non echo planar, diffusion-weighted magnetic resonance imaging for the detection of residual or recurrent middle-ear cholesteatoma. J Laryngol Otol 128: 599-603. Link: https://goo.gl/FD4FXx
- Pizzini FB, Barbieri F, Beltramello A (2010) HASTE diffusion-weighted 3-Tesla magnetic resonance imaging in the diagnosis of primary and relapsing cholesteatoma. Otol Neurotol 31: 596-602. Link: https://goo.gl/PtDbAV
- Garrido L, Cenjor C, Montoya J (2015) Capacidad diagnostica de la resonancia magnética con técnica de difusión no echo-planar en la detección de colesteatomas primarios y recurrentes. Acta Otorrinolaringol Esp 66: 199-204. Link: https://goo.gl/fzDPhR
- Karandikar A, Loke SC, Goh J (2015) Evaluation of cholesteatoma: our experience with DW Propeller imaging. Acta Radiol 56: 1108-1112. Link: https://goo.gl/FqZwnc
- Dubrulle F, Souillard R, Chechin D (2006) Diffusion-weighted MR imaging sequence in the detection of postoperative recurrent cholesteatoma. Radiology 238: 604-610. Link: https://goo.gl/wo8khP
- Rodríguez Artajejo (1990) Análisis de decisiones clínicas. Med Clin (Barc) 94: 348-354. Link: https://goo.gl/1NTfAE
- Migirov L, Tal S, Eyal A (2009) MRI, not CT, to rule out recurrent cholesteatoma and avoid unnecessary second-look mastoidectomy. Isr Med Assoc J 11: 144-146. Link: https://goo.gl/c9MXN4
- Lingam RK, Nash R, Majithia A (2016) Non-echoplanar diffusion weighted imaging in the detection of post-operative middle ear cholesteatoma: navigating beyond the pitfalls to find the pearl. Insights Imaging 7: 669-678. Link: https://goo.gl/R74vKC
- Henninger B, Kremser C (2017) Diffusion weighteg imaging for the detection and evaluation of cholesteatoma. World J Radiol 9: 217-222. Link: https://goo.gl/YWniEN
- Algin O, Aydin H, Ozmen E (2017) Detection of cholesteatoma: high-resolution DWI using RS-EPI and parallel imaging at 3 tesla. Journal Neuroradiology 44: 388-394. Link: https://goo.gl/u8Mb7g
- Yamashita K, Yoshiura T, Hiwatashi A (2011) Detection of middle ear cholesteatoma by diffusion-weighted MR imaging: multishot echo-planar imaging compared with single-shot echo-planar imaging. AJNR 32: 1915-1918. Link: https://goo.gl/Y9kXtc
- De Foer B, Vercruysse JP, Pilet B (2006) Single-shot, turbo spin-echo, diffusion-weighted imaging versus spin-echo-planar, diffusion-weighted imaging in the detection of acquired middle ear cholesteatoma. AJNR 27: 1480-1482. Link: https://goo.gl/BX2VPr
- Evlice A, Tarkan Ö, Kiroğlu M (2012) Detection of recurrent and primary acquired cholesteatoma with echo-planar diffusion-weighted magnetic resonance imaging. J Laryngol Otol 126: 670-676. Link: https://goo.gl/31abbz
- De Foer B, Vercruysse JP, Bernaerts A (2007) The value of single-shot turbo spin-echo diffusion-weighted MR imaging in the detection of middle ear cholesteatoma. Neuroradiology 49: 841-848. Link: https://goo.gl/yKQaeY
- Dhepnorrarat RC, Wood B, Rajan GP (2009) Postoperative non-echo-planar diffusion-weighted magnetic resonance imaging changes after cholesteatoma surgery: implications for cholesteatoma screening. Otol Neurotol 30: 54-58. Link: https://goo.gl/JpbMbt
- Venail F, Bonafe A, Poirier V (2008) Comparison of echo-planar diffusion-weighted imaging and delayed postcontrast T1-weighted MR imaging for the detection of residual cholesteatoma. AJNR 29: 1363-1368. Link: https://goo.gl/uQiz8Q
- Aikele P, Kittner T, Offergeld C (2003) Diffusion-weighted MI imaging of cholesteatoma in pediatric and adult who have undergone middle ear surgery. AJR 181: 261-265. Link: https://goo.gl/xxYh5w
- Vercruysse JP, De Foer B, Pouillon M (2006) The value of diffusion-weighted MR imaging in the diagnosis of primary acquired and residual cholesteatoma: a surgical verified study of 100 patients. Eur Radiol 16: 1461-1467. Link: https://goo.gl/mgJXct
- Clark MPA, Westerberg BD, Fenton DM (2010) The ongoing dilemma of residual cholesteatoma detection: are current magnetic resonance imaging technique good enough? J laringol Otol 124: 1300-1304. Link: https://goo.gl/pD8p1q
- Lingam RK, Basset P (2017) A meta-analysis on the diagnostic performance of non-echoplanar diffusion-weighted imaging in detecting middle ear cholesteatoma: 10 years on. Otol Neurotol 38: 521-528. Link: https://goo.gl/Gcc2t9
- Dremmen MH, Hofman PA, Hof JR (2012) The diagnostic accuracy of non-echo-planar diffusion-weighted imaging in the detection of residual and/or recurrent cholesteatoma of the temporal bone. AJNR 33: 439-444. Link: https://goo.gl/C3K8DS
- Blaney SP, Tierney P, Oyarazabal M (2000) CT scanning in "second look" combined approach tympanoplasty. Rev Laryngol Otol Rhinol (Bord) 121: 79-81. Link: https://goo.gl/x47ujD
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
