Archives of Otolaryngology and Rhinology
Choristoma of the vestibular nerve: Should it influence our management of vestibular Schwannoma- Case report and review of the literature
Talal Aboud1*, Ghaith Aboud2, Rami O Almefty3,4, Kaith K Almefty3,4, Rebecca D Folkerth5,6 and Ossama Al-Mefty3,6
2Virginia Tech Carilion School of Medicine, Roanoke, VA, USA
3Department of Neurosurgery, Brigham and Women's Hospital, USA
4Barrow Neurological Institute, Phoenix, Arizona, USA
5Department of Pathology (Neuropathology), Brigham and Women's Hospital, USA
6School of Harvard University, Boston MA, USA
Cite this as
Aboud T, Aboud G, Almefty RO, Almefty KK, Folkerth RD, et al. (2019) Choristoma of the vestibular nerve: Should it influence our management of vestibular Schwannoma- Case report and review of the literature. Arch Otolaryngol Rhinol 5(3): 091-094. DOI: 10.17352/2455-1759.000106Background and Importance: Choristomas of the internal auditory canal and cerebellopontine angle are rare, non-neoplastic lesions that mimic vestibular schwannomas and may subsequently be subject to treatment by surgical resection or radiosurgery. Their management is conservative with observation. Surgical intervention has been associated with expected hearing loss that is counter to the goal of surgery. On the other hand, radiosurgery is not indicated in such pathology and will also lead to eventual hearing loss.
Case Description: We report here a case of an intracanalicular choristoma that was diagnosed post-surgically that was expected a vestibular schwannoma. We highlight the differentiation and its implication on the management of intracanalicular lesions.
Conclusion: Since the lesions might contain fat, we recommend fat suppression sequences are obtained on MRI for all expected vestibular schwannomas with the quest to unveil pretreatment the diagnosis of choristoma. We also believe that heightening the awareness of their presence will encourage surgeons to abort resection after pathological confirmation. The majority of intracanalicular lesions today might be treated with radiosurgery without pathological diagnosis. With the lack of knowledge of the true incidence of choristomas, such practice will include exposing an unknown number of patients to the risk of radiosurgery.
Background and Importance
Choristomas of the internal auditory canal (IAC) and cerebellopontine angle (CPA) are rare benign lesions. Lipochoristomas are believed to represent 0.1% of all IAC/CPA tumors, however; this may be an underrepresentation as many IAC lesions are presumed to be vestibular schwannomas (VS) without pathologic confirmation [1-3].
Choristomas are considered developmental anomalies rather than neoplasms, which they may mimic on imaging and subject the patient to a treatment that will not be beneficial and carries risk. For this reason, choristomas in the IAC should be distinguished from schwannomas, which are the most common type of tumors in this location. Ideally, and it is possible in some cases using specific MRI sequences, choristoma could be distinguished pretreatment. With high awareness of their features, one might abort resection if they were unexpectedly encountered at surgery. Their presence and real incidence raises reservation about treating intracanalicular lesions with radiosurgery without pathological diagnosis. We present a case of an IAC choristoma that was treated surgically, review the literature, discuss the available means to identify it before surgery, heighten the awareness of their features so they can be recognized if they were found unexpectedly during surgical exposure, and raise the reservations of treatment for intracanalicular lesions with radiosurgery without pathological confirmation.
Clinical Presentation
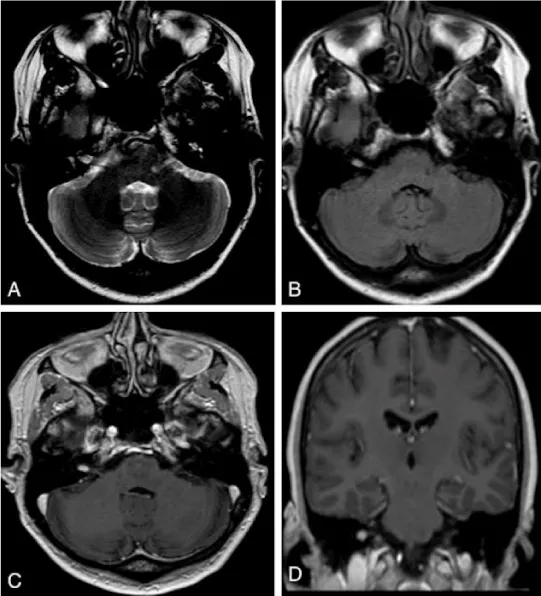
A 55-year-old left-handed physician presented with progressive vertigo over one year that was intolerable, and refractory to vestibular therapy, Epley maneuver, or steroids. An audiogram did not reveal any sensorineural hearing loss. The patient denied tinnitus, otalgia and noise exposure. An MRI showed a right-sided, homogenously enhancing 5.5 mm lesion in the IAC along the 7th/8th nerve complex consistent with a VS (Figure 1), there was no apparent precontrast T1 hyperintense lesion.
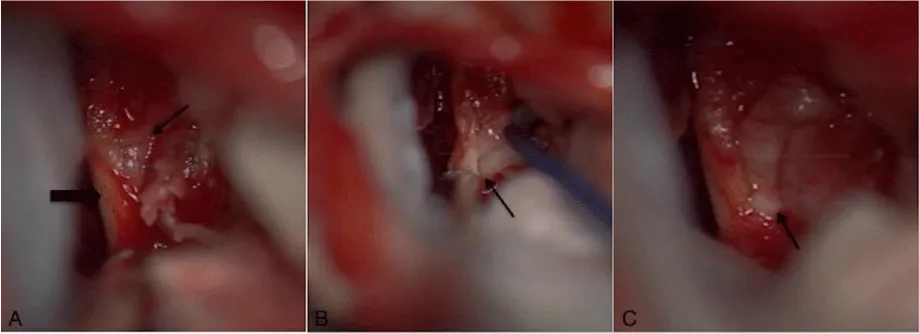
Based on the presumed diagnosis of VS, treatment options were thoroughly discussed with this highly informed and knowledgeable patient with an emphasis on observation. As the patient’s symptoms persisted, she was decisive in proceeding with surgical resection. After obtaining informed consent, a transmastoid retrosigmoid approach [4] with intraoperative monitoring was performed. The IAC was drilled and the tumor was exposed. A grayish yellow tumor-like tissue protruded to the surface separating the vestibular nerves (Figure 2). Microsurgical technique was utilized to resect the mass while preserving all of the main nerve branches in the meatus. However, there was no cleavage plane at all at the lateral pole of the tumor as it merged into the nerve fibers. Unfortunately, the brainstem auditory evoked response (BAER) recording was lost prior to closure and after the completion of the resection. The patient had an uneventful recovery but unfortunately has not regained serviceable hearing. Her dizziness has persisted, and she is being considered for a chemical labyrinthectomy. Three month follow-up MRI showed no evidence of residual or recurrent tumor with enhancement extending from the IAC to the basal turn of the cochlea consistent with a possible labyrinthitis.
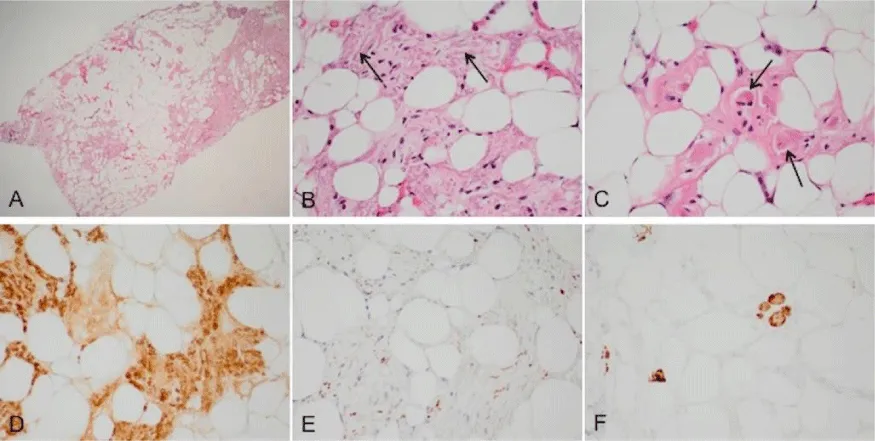
Light microscopy of hematoxylin-and-eosin (H&E)-stained slides showed disorganized aggregates of myelinated nerve fibers, adipose tissue, scattered skeletal muscle cells and collagen (Figure 3). Immunohistochemistry using standard techniques confirmed the presence of these cell types. There was no evidence of malignancy or Schwann cell proliferative process (i.e., schwannoma).
Discussion
Choristomas are rare benign lesions that may arise in virtually any location. Rarely, they may be found intracranially, particularly in the cerebellopontine angle (CPA), internal auditory canal (IAC), and optic nerves and chiasm [5,6]. While hamartomas are abnormal proliferations of normal tissue in locations where the tissue is normally found, choristomas arise in abnormal locations for the proliferating tissue [5]. Several types of histologic patterns have been reported, such as neuromuscular choristoma [7], smooth muscle choristoma [8] and lipochoristoma [9,10], depending on the dominant tissue type composing the mass. Reaching a definite diagnosis may be challenging for tumor locations like the CPA and IAC as it requires a skull base approach to obtain adequate tissue. In cases of non-neoplastic lesions that have an indolent course such as choristomas for which the preferred management is observation and symptomatic treatment, reaching a diagnosis noninvasively becomes crucial in order to avoid unnecessary surgical excision or radiosurgery.
Reported surgical resection of choristoma, though curative, is associated with late complications such as hearing loss, facial paralysis and vestibulopathies [1,7,8,]. An accurate estimate of the rate of complications is not available due to the lack of sufficient data on such rare lesions, but the available literature suggests a high incidence of post-operative hearing loss which is not surprising considering their pathogenesis. It is suggested that embryonic remnants present as integral components of CNVIII are the origin of choristomas in the IAC [1,11,12]. While preservation of hearing is the aim and justification of operating on lesions limited to the IAC. Likewise, the experience of treating with radiosurgery intracanicular masses indicates a definitive progressive hearing loss despite the early preservation immediately post treatment. Multiple studies have shown a progressive, definitive long term decline to non-functional hearing following radiosurgery [13-16], Hearing is lost from damage to the cochlear nerve at the level of the cochlea and this has been shown to occur when the cochlea receives a dose over 3 Gy [15,17], In one study, only those patients who received a dose of less than 2 Gy to the cochlea preserved hearing.18 According to Linskey et. al. calculations, the cochlea typically receives a dose of 5-8 Gy for vestibular schwannomas [19].
The most effective non-invasive tool to study these lesions is MRI. Various MRI sequences provide useful information to aid in the detection of the various tissue types composing the mass. Neuromuscular and smooth muscle choristomas are isointense and enhance strongly with contrast [7,8], whereas lipochoristomas are hyperintense and do not usually enhance with contrast. The most effective method to detect them is by comparing the MRI with and without fat suppression, which will cause a signal loss in choristomas with high lipid content [9,10]. This concept is helpful with lipomas of the CPA and IAC, as the treatment of choice of these lesions is observation as well [20]. Nonetheless, in many occasions, choristomas are not distinguishable from VS [7] and the diagnosis is made after resection of the mass. The point of preoperative diagnosis is particularly important because of the high number of IAC tumors which are treated with radiosurgery without pathologic diagnosis. According to Carlson et al. in a review of 8330 patients treated between 2004 and 2011, radiosurgery was used without pathologic diagnosis in 29% of presumed VSs [21], and likely most if not all strictly intracanalicular tumors are treated without pathologic diagnosis. Clearly, there is no indication for radiosurgery for benign developmental lesions such as choristomas and exposes patients to the known and potential risks of radiosurgery and almost certain eventual hearing loss [13-16].
When MRI is non-diagnostic in a patient with a symptomatic IAC mass, and surgery is commenced, the surgeon should have a heightened awareness and intraoperative pathologic consultation can guide the surgical goals [1]. In the event that a choristoma is encountered, resection can be aborted with the goal of neurologic preservation.
Conclusion
Choristomas are rare benign mass lesions that can occur in the IAC and mimic VSs. Choristomas are best treated conservatively, are unlikely to require resection and have no expected benefit from radiation. Since hearing loss rather than hearing preservation might be induced by surgical resection, one should refrain from complete surgical resection. On the other hand, the high number of IAC tumors treated primarily with radiosurgery today without pathologic confirmation, the occurrence of choristomas raise issues with this strategy, particularly because the exact incidence of intracanalicular choristomas are unknown. Identifying these lesions based on MRI could prevent many patients from unnecessary treatment. We suggest that fat suppression can indicate the presence of a choristoma or lipoma rather than a VS and should be performed in the evaluation of IAC lesions. In the event that a preoperative diagnosis is not possible, awareness of these lesions can allow for intraoperative pathology to better define the surgical goals.
- Wu SS, Lo WW, Tschirhart DL, Slattery WH, Carberry JN, et al. (2003) Lipochoristomas (lipomatous tumors) of the acoustic nerve. Arch Pathol Lab Med 127: 1475-1479. Link: http://bit.ly/2BAQgpg
- Scangas G, Remenschneider A, Santos F (2015) Lipochoristoma of the Internal Auditory Canal. J Neurol Surg Rep 76: e52-e54. Link: http://bit.ly/3484uKD
- Smith MM, Thompson JE, Thomas D (1997) Choristomas of the seventh and eighth cranial nerves. AJNR Am J Neuroradiol 18: 327-329. Link: http://bit.ly/2BKdM2V
- Abolfotoh M, Dunn IF, Al-Mefty O (2013) Transmastoid retrosigmoid approach to the cerebellopontine angle: surgical technique. Neurosurgery 73: 16-23. Link: http://bit.ly/32JJQQD
- Kazim M, Kennerdell JS, Maroon J, Rothfus W, Marquardt M (1992) Choristoma of the optic nerve and chiasm. Arch Ophthalmol 110: 236-238. Link: http://bit.ly/31N2SV3
- Zimmerman LE, Arkfeld DL, Schenken JB, Arkfeld DF, Maris PJ (1983) A rare choristoma of the optic nerve and chiasm. Arch Ophthalmol 101: 766-770. Link: http://bit.ly/31JtMwW
- Nikolaou G, Röösli C, Huber A, Probst R (2012) Neuromuscular choristoma of the internal auditory meatus. ORL J Otorhinolaryngol Relat Spec 74: 246-249. Link: http://bit.ly/2BCeoI0
- Lassaletta L, Granell J, Patrón M, Gavilán J (2005) Smooth muscle choristoma of the internal auditory meatus. Eur Arch Otorhinolaryngol 262: 834-838. Link: http://bit.ly/2Wec03B
- De Diego B, Trinidad A, Ibáñez A, Saucedo G, Vicente J, et al. (2012) Ramírez-Camacho R. Lipochoristoma of the internal auditory canal. B-ENT 8: 295-297. Link: http://bit.ly/32KuL0X
- DeGregoris G, Brumblay H, Arts H, Thompson BG (2007) Lipochoristoma of the cerebellopontine angle. Otol Neurotol 28: 867-868. Link: http://bit.ly/2BGStj4
- Fekete DM, Wu DK (2002) Revisiting cell fate specification in the inner ear. Curr Opin Neurobiol 12: 35-42. Link: http://bit.ly/33UPPSP
- Lang H, Fekete DM (2001) Lineage analysis in the chicken inner ear shows differences in clonal dispersion for epithelial, neuronal, and mesenchymal cells. Dev Biol 234: 120-137. Link: http://bit.ly/2BKeXiR
- Carlson ML, Jacob JT, Pollock BE (2013) Long-term hearing outcomes following stereotactic radiosurgery for vestibular schwannoma: patterns of hearing loss and variables influencing audiometric decline. J Neurosurg 118: 579-587. Link: http://bit.ly/342xq6F
- Chopra R, Kondziolka D, Niranjan A, Lunsford LD, Flickinger JC (2007) Long-term follow-up of acoustic schwannoma radiosurgery with marginal tumor doses of 12 to 13 Gy. int J radiat oncol Biol Phys 68: 845-851. Link: http://bit.ly/2PgVn5U
- Hasegawa T, Kida Y, Kato T, Iizuka H, Yamamoto T (2011) Factors associated with hearing preservation after Gamma Knife surgery for vestibular schwannomas in patients who retain serviceable hearing. J Neurosurg 115: 1078-1086. Link: http://bit.ly/32L9a8W
- Roos DE, Potter AE, Brophy BP (2012) Stereotactic radiosurgery for acoustic neuromas: what happens long term? int J radiat oncol Biol Phys 82: 1352-1355. Link: http://bit.ly/2BD9BGh
- Tamura M, Carron R, Yomo S (2009) Hearing preservation after gamma knife radiosurgery for vestibular schwannomas presenting with high-level hearing. Neurosurgery 64: 289-296. Link: http://bit.ly/2odqCDP
- Baschnagel AM, Chen PY, Bojrab D (2013) Hearing preservation in patients with vestibular schwannoma treated with Gamma Knife surgery. J Neurosurg 118: 571-578. Link: http://bit.ly/2BAOF2N
- Linskey ME, Johnstone PA, O’Leary M, Goetsch S (2003) Radiation exposure of normal temporal bone structures during stereotactically guided gamma knife surgery for vestibular schwannomas. J Neurosurg 98: 800-806. Link: http://bit.ly/2PcvV1k
- White JR, Carlson ML, Van Gompel JJ (2013) Lipomas of the cerebellopontine angle and internal auditory canal: Primum Non Nocere. Laryngoscope 123: 1531-1536. Link: http://bit.ly/2pLot2G
- Carlson ML, Habermann EB, Wagie AE (2015) The Changing Landscape of Vestibular Schwannoma Management in the United States-A Shift Toward Conservatism. Otolaryngol Head Neck Surg 153: 440-446. Link: http://bit.ly/2MKvZUG
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
