Archives of Otolaryngology and Rhinology
Quantitative Analysis of Smooth Pursuit Eye Movement Using Video-Oculography
Hironori Fujii, Makoto Hashimoto, Kazuma Sugahara, Takuo Ikeda, Yoshinobu Hirose and Hiroshi Yamashita*
Cite this as
Fujii H, Hashimoto M, Sugahara K, Ikeda T, Yamashita H (2018) Quantitative Analysis of Smooth Pursuit Eye Movement Using Video-Oculography. Arch Otolaryngol Rhinol 4(1): 030-034. DOI: 10.17352/2455-1759.000071Background: Abnormalities of smooth pursuit eye movement (SPEM) are a clinical finding in central equilibrium disorders; the evaluation of SPEM using video-oculography (VOG) has therefore been conducted clinically in recent years. However, evaluation criteria for determining a saccadic pattern have yet to be clearly defined.
The context and purpose of the study: Here, we report SPEM using VOG performed with commercially available equipment. The results were then quantitatively evaluated.
Methods: Twelve patients treated at the Department of Otolaryngology at our hospital underwent SPEM testing using both electronystamography (ENG) and VOG. Eye movements were binarized using ImageJ software; these data were used for statistical analysis. Evaluation criteria included the number of saccadic eye movements, average eye movement velocity, average difference between target and eye movement velocities, and phase lag between target and eye movements.
Results: We examined a healthy pattern group (n=6) and saccadic pattern group (n=6). A significant difference between the healthy and saccadic pattern groups was identified in the number of saccadic eye movements, average eye movement velocity, and average difference between target and eye movement velocities.
Conclusion: Quantitative evaluation of SPEM using VOG was possible, and it facilitated the identification of useful evaluation criteria.
Potential implication: As SPEM testing can non-invasively evaluate brain stem and cerebellar function, screening is possible using this simple method.
Introduction
Abnormalities of smooth pursuit eye movement (SPEM) are a clinical finding in central equilibrium disorders. SPEM maintains a stable image of an object on the fovea, recruiting the visual cortex, medial temporal area, medial superior temporal area (MST), frontal eye field (FEF), cerebellum, vestibular nucleus, and ophthalmic nucleus [1-3].
SPEM can be measured using two methods
Electronystamography (ENG) and video-oculography (VOG). ENG employs electrodes to record eye movement using the change in retinal-corneal potential. VOG is a method of recording the position of the pupil and calculating its angle with an infrared CCD camera. When comparing eye movement records taken by ENG and VOG, the latter has superior features: good spatial resolution, accurate recording of vertical components, and provides technical simplicity when performing recordings. SPEM measurement using VOG has been previously reported as a useful method for examinations of head trauma and stroke, in which quantitative analysis was used to compare patient and healthy groups [4,5], and for comparing findings between multiple sclerosis and Parkinson's disease [6]. However, VOG is considered relatively expensive [7].
We analyzed ocular movements using a novel VOG system using a commercially available CCD camera, personal computer, and free image software (ImageJ, National Institutes of Health [NIH], Maryland, USA). We investigated whether quantitative evaluation of SPEM is possible by applying the VOG system to identify parameters that distinguish a healthy pattern from a saccadic pattern of SPEM.
Materials and Methods
Patients and controls
Patients who were diagnosed as having either a healthy pattern or saccadic pattern of SPEM using ENG were retrospectively evaluated using VOG. We conducted simultaneous recordings with ENG and VOG of six subjects in both groups at Yamaguchi University Hospital. Distinguishing between healthy and saccadic patterns based on ENG findings was qualitatively decided based on the consensus of specialists with an average of more than 5 years of clinical experience. This study was approved by Yamaguchi University School of Medicine Ethics Committee and corresponds to the simplification of formal consent per Chapter 5 of the Ethics Guidelines regarding people.
Recording of eye movements
The examination was performed in a dark room, where the subjects sat down and placed their head on a chin rest. Using the ENG optotype stimulator, an indicator appeared on the surface of a 50 × 50 cm planar device (Nagashima, Tokyo, Japan). The stimulation was performed using a sine wave. The target moved at a frequency 0.25 Hz and a horizontal amplitude of 30°. The maximum velocity of the visual target was 47.1°/sec. The subject’s eye movements were recorded using an infrared CCD camera (ET 60 LW 2, New Opto, Kanagawa, Japan) with a half mirror for viewing the target. Horizontal eye movements in the right eye were captured at a sampling rate of 30 Hz using the VOG system. The video of eye movements taken by the infrared CCD camera was sent to one channel of a screen dividing unit (Ikegami, Japan). Footage of the target was recorded with a video camera and was sent to another channel of the screen dividing unit. The screen division unit synthesized an image that was then sent to an analog-to-digital (AD) converter. The digitized file was transferred to a personal computer (PC; MacBook Pro, Apple, California, USA) using a firewire interface from the AD converter. QuickTime Pro 7 (Apple, California, USA) was used as the recording software. We used a sampling rate of 30 frames per second. Images imported using QuickTime Pro were analyzed and quantified using the image analysis software, ImageJ (National Institutes of Health, Bethesda, MD, USA) [8]. By binarizing the pupil data collected in the ETT examination and extracting the movement of the pupil’s center every 1/30 sec, we obtained the coordinates of the pupil’s center to analyze eye movements. There are reports of three-dimensional analyses using this system [9,10]; however, this study was conducted in two dimensions.
Eye movement analysis
Calibration was conducted in a test that involved gazing at a target located at 30° extremes. An average of the coordinates that would require a specific subject to gaze 30° rightwards was set on the VOG; the visual target was then presented on the device at the established location 30° to the right. The same procedure was performed in the opposite direction to calibrate measurements on the left side. Using the obtained values, coefficients for the transformation from the position coordinates collected from the SPEM examination to the angle coordinates of the eyeball were calculated. We used the coefficients to convert the position coordinates of the eye movement and visual target into angles. We then analyzed a waveform without artefacts, such as blinking, from the time when the target was at the value closest to 0 for the following 120 frames (i.e., 4 seconds). The velocity was obtained by dividing the difference between the coordinates of a given position and the coordinates of a position 0.03 seconds later by 0.03.
Evaluation criteria and calculation formula
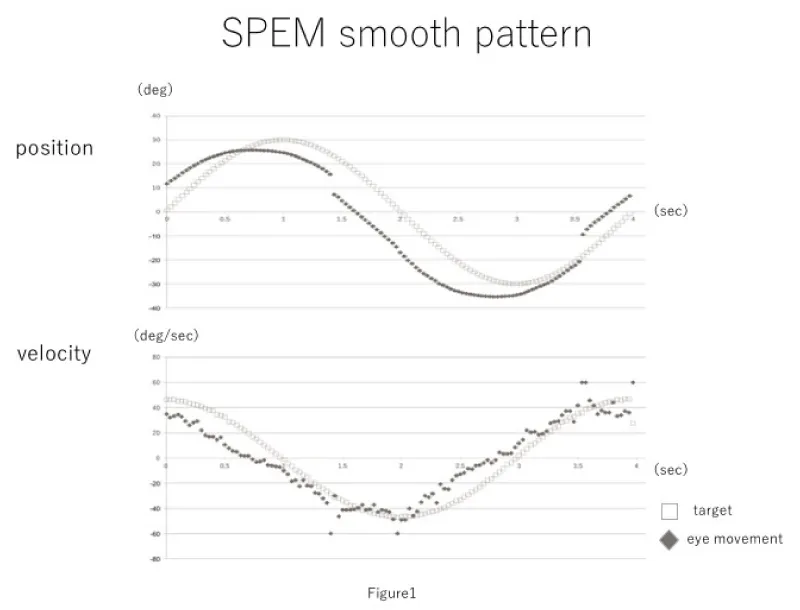
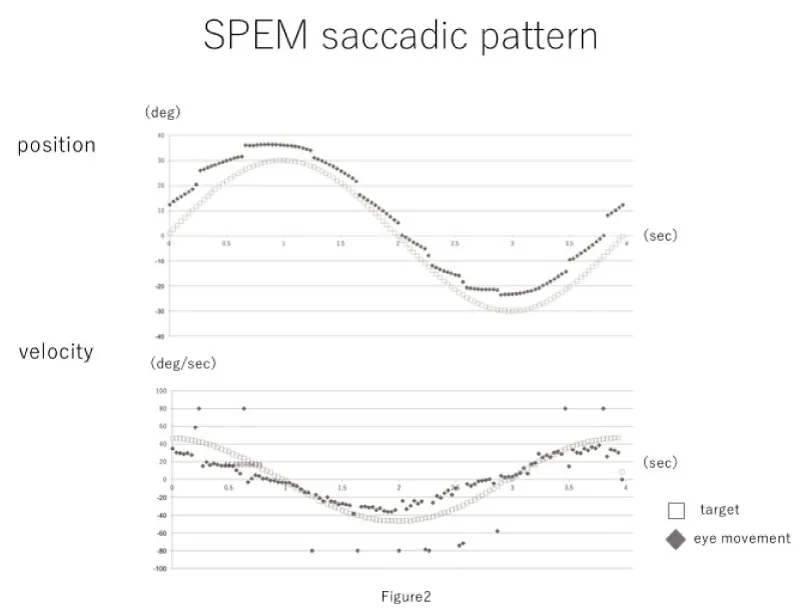
Evaluation criteria included the number of saccadic eye movements, average eye movement velocity, average difference between the target and eye movement velocities, and the phase lag between the target and eye positions. The obtained waveforms are shown in figures 1,2.
1. Number of saccadic eye movements in one time period: Eye movements with a velocity ≥ 80°/sec within one (33 ms) to two frames (67 ms) were categorized as “saccadic eye movements” based on the methodology of a previous study [11]. We set the cut-off value to approximately twice the maximum value of the eye movement, and allowed for an interval period of no more than 1 second. If the saccadic eye movement lasted more than three frames, it was excluded as an artefact.
2. Average eye movement velocity: The absolute values of the velocity were added together and divided by the number of frames. We excluded frames that included saccadic eye movement.
3. Average difference between target and eye movement velocities: We calculated the average absolute difference between the target and eye movement velocities. The absolute numerical value was summed, and the average velocity was obtained by dividing it by the number of frames. As in the analysis of the average eye movement velocity, we excluded frames that included saccadic eye movement.
4. Phase lag: The waveform of the eye position was translated parallel to the x-axis (time) such that the number of frames between its location and the target position wave form was minimized. The number of frames required for the translation was taken as the phase difference. One full cycle of the waveform, buffered by one half-cycle at either end, was used for analysis of criteria 1 through 3.
Results
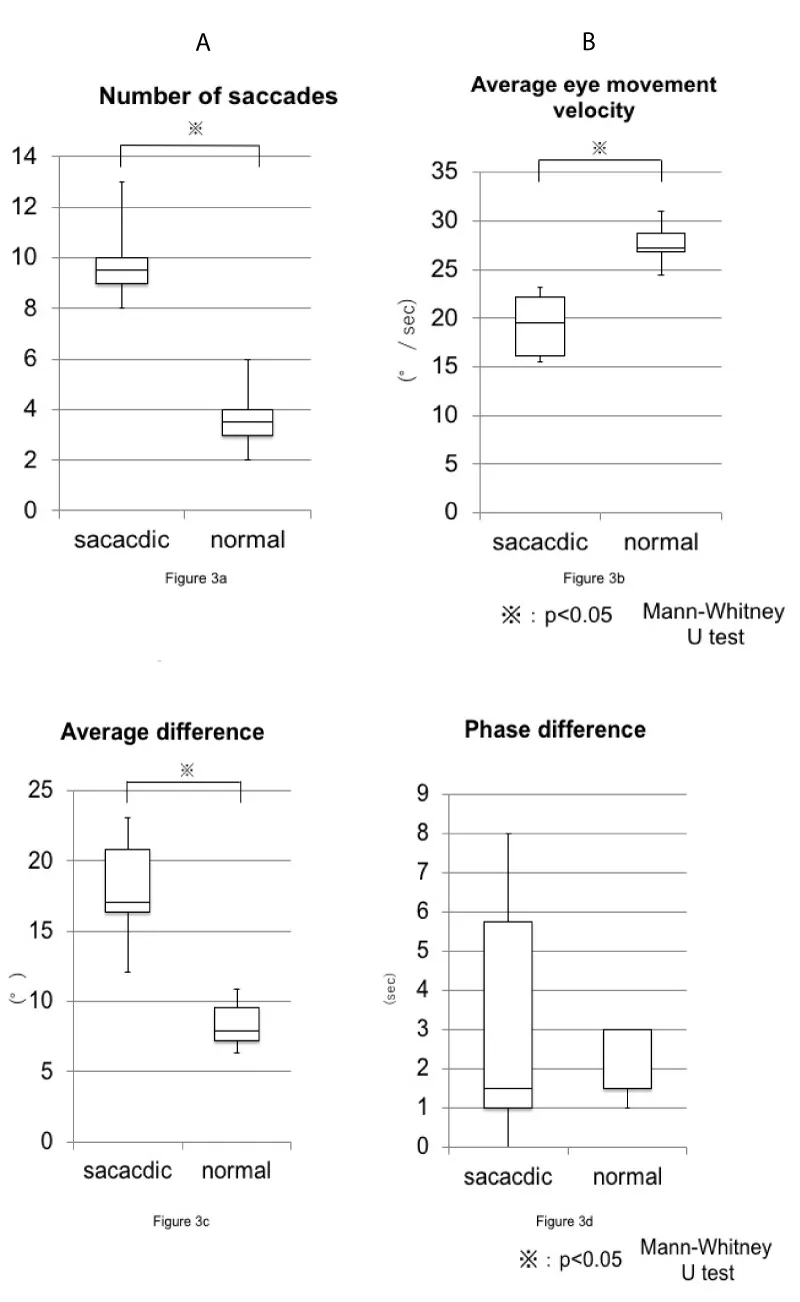
There were six patients in the healthy pattern group and six patients in the saccadic pattern group. The mean age of the healthy pattern group was 56.3 years (median, 58 years; min, 38 years; max, 76 years), and that of the saccadic group was 50.6 years (median, 55.5 years; min, 13; max, 69). Primary diseases in the saccadic pattern group included spinocerebellar degeneration, unilateral semi-circular canal paralysis, progressive supranuclear palsy, brain stem tumor surgery, multiple sclerosis, and Chiari malformation. The mean number of saccades was 3.6 ± 1.2 (median, 3.5; min, 2; max, 6) and 9.7 ± 1.4 (median, 9.5; min, 7; max, 13) for the healthy pattern group and the saccadic pattern group, respectively. The average eye movement velocity was 27.6 ± 1.6°/sec (median, 27.2°/sec; max, 31.0°/sec; min, 24.5°/sec) in the healthy pattern group, and 19.3 ± 2.5°/sec (median, 19.0°/sec; max, 23.2°/sec; min, 15.5°/sec) in the saccadic group. The difference between the target and eye movement velocities was 8.4 ± 1.2 °/sec (median, 7.9°/sec; max, 11.0°/sec; min, 6.4°/sec) in the healthy pattern group and 17.9 ± 3.0°/sec (median, 17.1°/sec; max, 23.1°/sec; min, 12.1°/sec) in the saccadic pattern group. The phase difference was 2.3 ± 0.75 frames in the healthy pattern group and 3.2 ± 2.5 frames in the saccadic pattern group (Table 1). Mann-Whitney's U tests were performed using the aforementioned results. The number of saccadic eye movements in one time period, average eve movement velocity, and average difference between target velocity and eye movement velocity were significantly different between the healthy pattern group and saccadic pattern group; the phase lag was not significantly different between groups (Figure 3).
Discussion
As VOG has been commonly used in the diagnosis of vertigo, we speculated that VOG had potential in diagnosing SPEM. From the results of this study, it can be concluded that evaluating SPEM using VOG is useful.
To diagnose SPEM, an examiner assesses a waveform (subjectively and qualitatively) taken from an ENG or VOG and determines whether it is indicative of either a healthy or saccadic movement pattern. This method has a clear disadvantage: as the results may differ depending on the evaluator, it is ultimately subjective. On the contrary, if the test was quantitatively evaluated, the results would be consistent and independent of the individual who evaluates the results. This study quantitatively evaluated individuals divided by a specialist according to whether they demonstrated a healthy or saccadic pattern; the study found a significant difference between the two groups. This suggests that by quantifying the test results, it is possible to objectively distinguish between the healthy pattern group and saccadic pattern group at a standard equivalent to traditional qualitative evaluation. In addition, quantitative evaluation would help to determine the degree of the saccadic pattern: it is possible to characterize the pathological condition by the number of saccadic movements in one waveform, the average velocity, and so forth. If the examination result deteriorates due with the progression of the disease state, it is possible to judge the degree of disease progression using the VOG assessment of SPEM. Qualitative evaluation would not suffice to discern the degree of the test results.
Professional equipment is usually necessary to perform quantitative analysis of SPEM. Though such devices can be purchased, and published studies have reported using them [12], they are expensive and impractical for routine use during medical treatment. Our investigation, however, used commercially available equipment and free software to construct a cheap, effective system: a commercially available infrared CCD camera and a PC. The cost-effectiveness of our method would make it easy to introduce into daily clinical practice. As much of the diagnostic equipment on sale has yet to be used in a published study, it is difficult compare our system with such commercial devices.
Our system is, however, marred by the possible disadvantage of capturing target and eye movements at only 30 Hz; it may be insufficient to cope with rapid eye movements. In order to evaluate the rapid eye movement using VOG, recording at 250 to 500 Hz is required [13,14]. In this study, evaluation was performed by setting the evaluation criteria for the SPEM, excluding the site where rapid eye movements occur. As a result, a significant difference was obtained between the groups, suggesting it is possible to evaluate SPEM even with a system that performs at 30 Hz.
Among the four evaluation criteria examined in this study, a statistically significant difference between the two groups was found for the number of saccadic eye movements in one time period, average eye movement velocity, and average difference between target velocity and eye movement velocity. Regarding the average difference between target velocity and eye movement velocity, there were significantly more cases in the group diagnosed as having a saccadic pattern using ENG findings. We set the cut-off value to about twice the maximum value of the eye movement and a consecutive period of no more than 1 second. The detection of rapid eye movement in SPEM was sufficient using this method. Furthermore, the number of impulsive eye movements was small but was observed in both groups. This suggests the possibility that impulsive eye movements – possibly psychological in origin - are performed during SPEM of healthy subjects without an underlying pathology.
Excluding the impulsive eye movements, the average velocity of eye movement among patients with a saccadic pattern tended to decrease across the experiment. Declining cerebellar function may cause the slowing of SPEM, as it would prevent the eyeball from following the target.
In the saccadic pattern group, the difference between the velocity of the target and the velocity of the eye movement increased across the experiment. Cerebellar dysfunction likely underlies both the increased difference, as well as the development of impulsive eye movements as a compensatory measure.
Regarding phase lag, there was no significant difference between the two groups in this study. This may be because the phase fluctuated greatly in each case; factors such as age may have confounded the effect from pathophysiology.
One of the limitations of the study might be the protocol using conventional CCD camera. If an ultrahigh-speed camera was used in this study, you can evaluate the rapid phase of eye movement could be evaluated. However, the camera used in this study is easily available. Therefore, our results of the research will contribute to the diagnosis in the general clinic.
Conclusion
SPEM testing using VOG can non-invasively evaluate brain stem and cerebellar function [15]. Particularly in regard to degenerative diseases, if it is possible to detect some abnormalities more sensitively and with greater ease than the use of imaging techniques allows for, our method may prove useful as a screening test. However, several problems persist: specialized equipment, such as a visual target presentation device, VOG, and equipment for performing ENG, is necessary and there are no established evaluation criteria to employ. Our findings suggest a solution to these difficulties; they provide feasible evaluation criteria that can be fulfilled by quantitative assessments using commercially available equipment and objective automatic discrimination.
We have confirmed with all the authors that there are no conflicts of interest to disclose.
- Strupp M, Kremmyda O, Adamczyk C, Böttcher N, Muth C, et al. (2014) Central ocular motor disorders, including gaze palsy and nystagmus. J Neurol 2: 542–558. Link: https://goo.gl/6yrKjS
- Robinson DA (1965) The mechanics of human smooth pursuit eye movement. J Phys 180: 569–591. Link: https://goo.gl/X7oZmy
- Leigh RJ, Zee DS (2006) The neurology of eye movements: Smooth pursuit and visual fixation, 4th Ed. Oxford University Press.
- Cifu DX, Wares JR, Hoke KW, Wetzel PA, Gitchel G, et al. (2015) Differential eye movements in mild traumatic brain injury versus normal controls. J Head Trauma rehabil 30: 21–28. Link: https://goo.gl/VfPqRP
- Johkura, K, Kawabata Y, Amano Y, Kudo Y, Murata H, et al. (2015) Bedside evaluation of smooth pursuit eye movements in acute sensory stroke patients. J Neurol Sci 348: 269–271. Link: https://goo.gl/qbxkkQ
- Pinkhardt EH, Kassubek J, Sussmuth S, Ludolph AC, Becker W, et al. (2009) Comparison of smooth pursuit eye movement deficits in multiple system atrophy and Parkinson's disease. J Neurol 256: 1438–1446. Link: https://goo.gl/DgP8bv
- McCaslin DL (2012) Electronystamograhpy and Videooculogrphy. Plural publishing.
- Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675. Link: https://goo.gl/hLC7Tn
- Tsutsumi T, Ikeda T, Fukuoka Y, Watanabe K, Kikuchi S (2012) Time course of the recovery of three-dimensional eye position in patients with acute cerebellitis. Auris Nasus Larynx 39: 540–543. Link: https://goo.gl/SB5Wts
- Tsutsumi T, Ikeda T, Fukuoka Y, Watanabe K, Kikuchi S (2013) Optokinetic stimulation can break Listing's law without induction of eye movement. Auris Nasus Larynx 40: 343–347. Link: https://goo.gl/Qu9C2u
- DiCesare CA, Kiefer AW, Nalepka P, Myer GD (2017) Quantification and analysis of saccadic and smooth pursuit eye movements and fixations to detect oculomotor deficits. Behav Res Methods 49: 258–266. Link: https://goo.gl/eZFE7w
- Hainline C, Rizzo JR, Hudson TE, Dai W, Birkemeier J, et al. (2017) Capturing saccades in multiple sclerosis with a digitized test of rapid number naming. J Neurol 264: 989–998. Link: https://goo.gl/1cTgYa
- Rizzo JR, Hudson TE, Dai W, Birkemeier J, Pasculli RM, et al. (2016) Rapid number naming in chronic concussion: eye movements in the King-Devick test. Ann Clin Transl Neurol 3(10): 801–811. Link: https://goo.gl/wXgAXG
- Moss HE, McCluskey L, Elman L, Hoskins K, Talman L, et al. (2012) Cross-sectional evaluation of clinical neuro-ophthalmic abnormalities in an amyotrophic lateral sclerosis population. J Neurol Sci 314(1-2): 97–101. Link: https://goo.gl/hEoRKp

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
